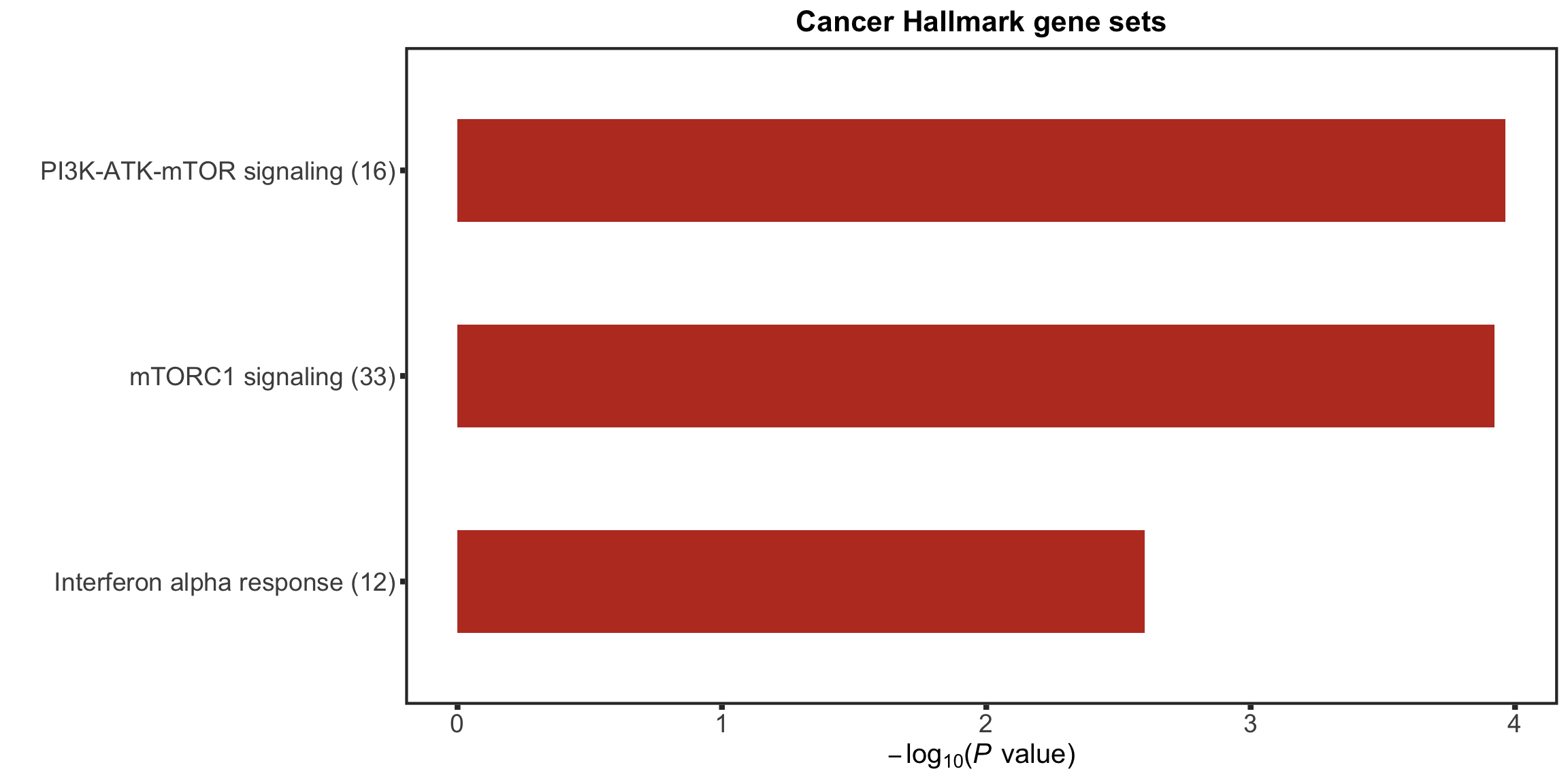
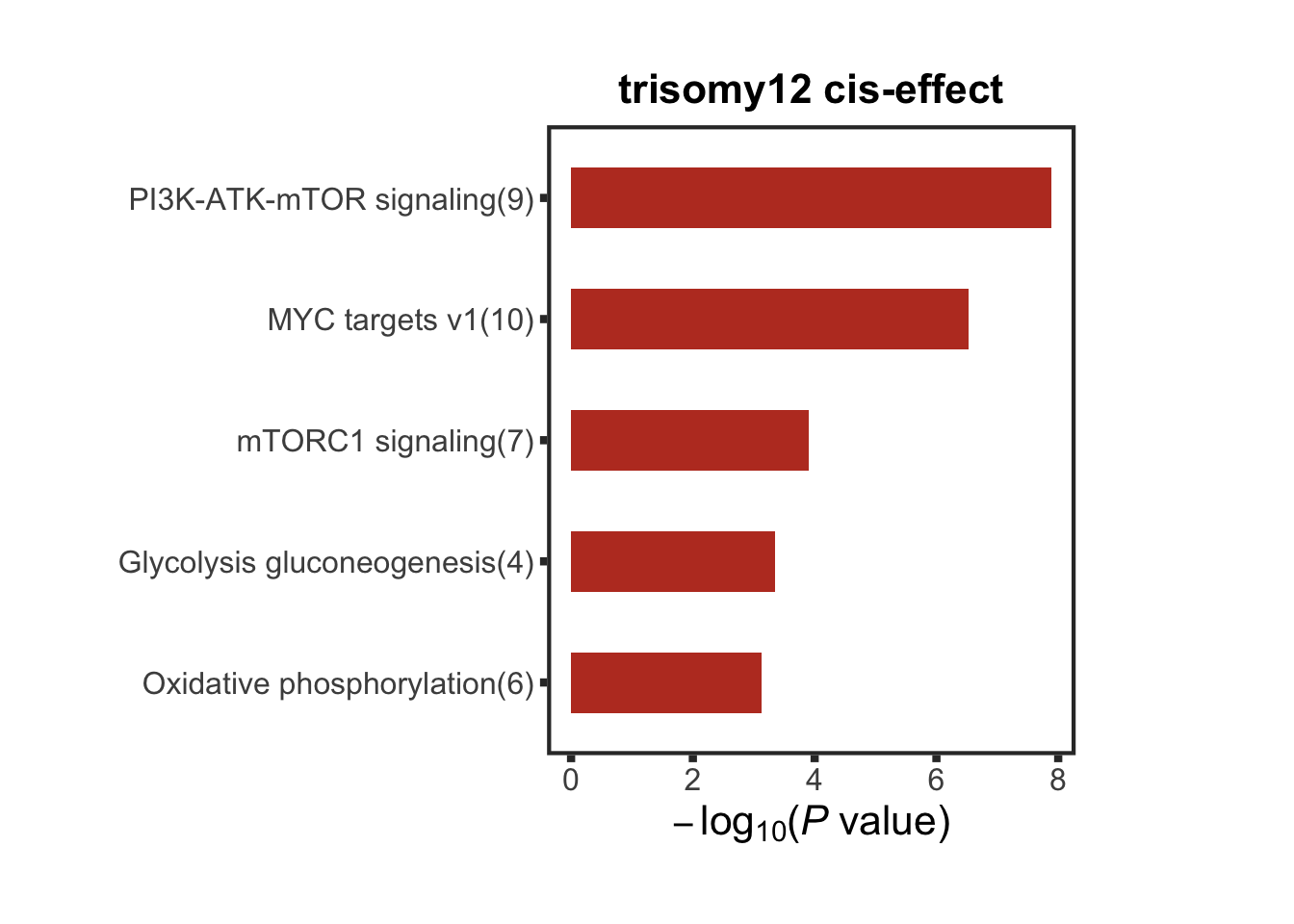
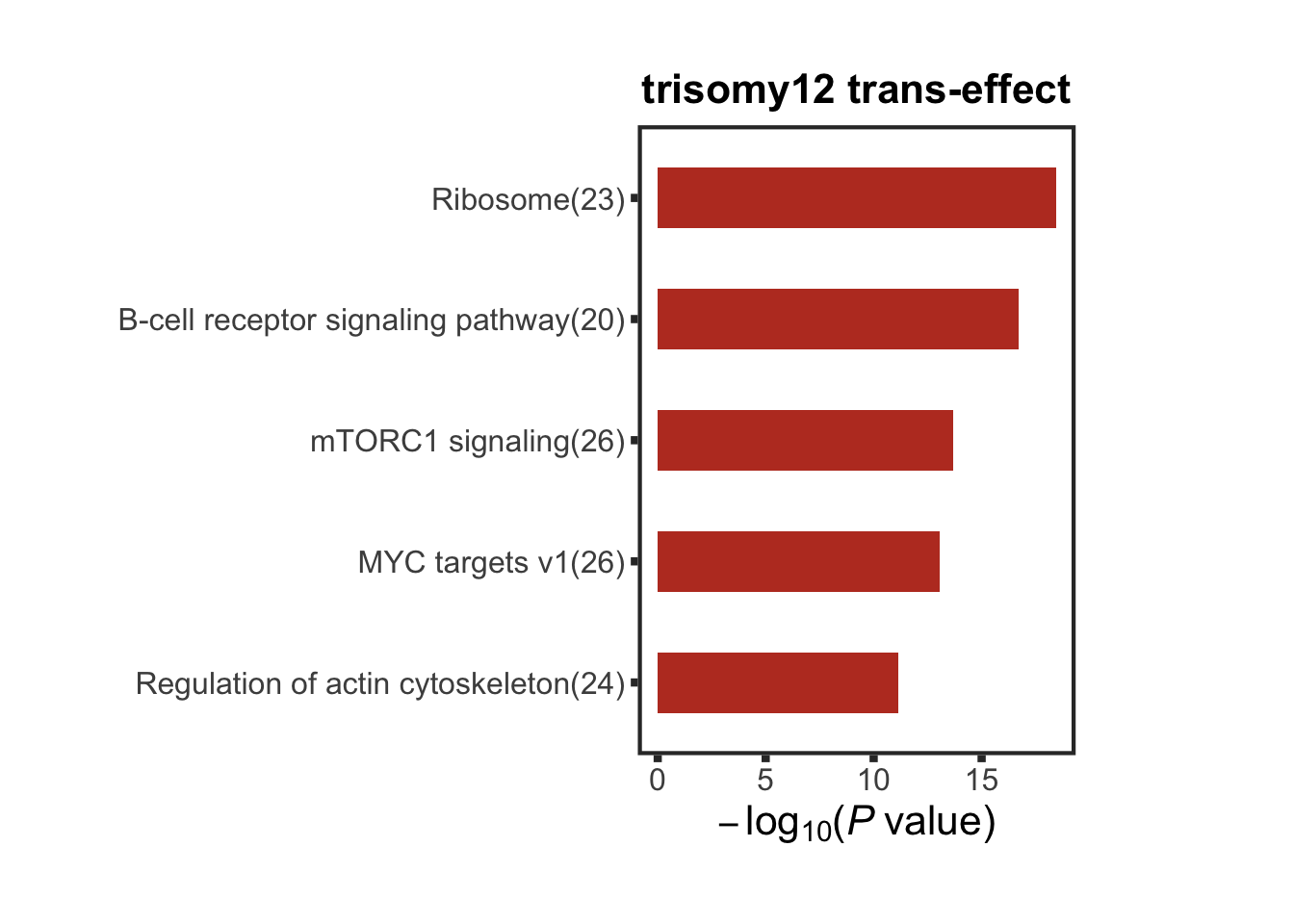
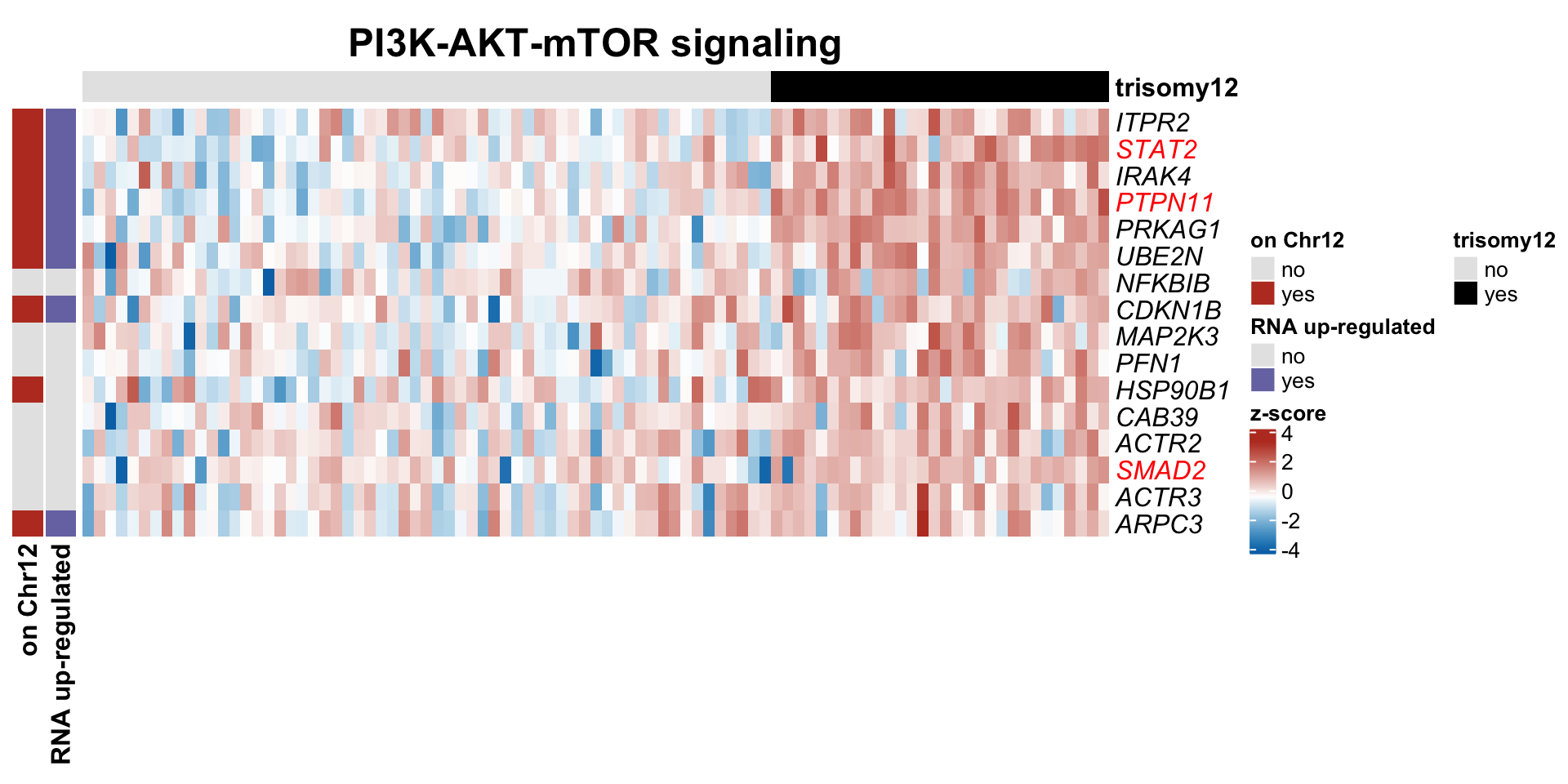
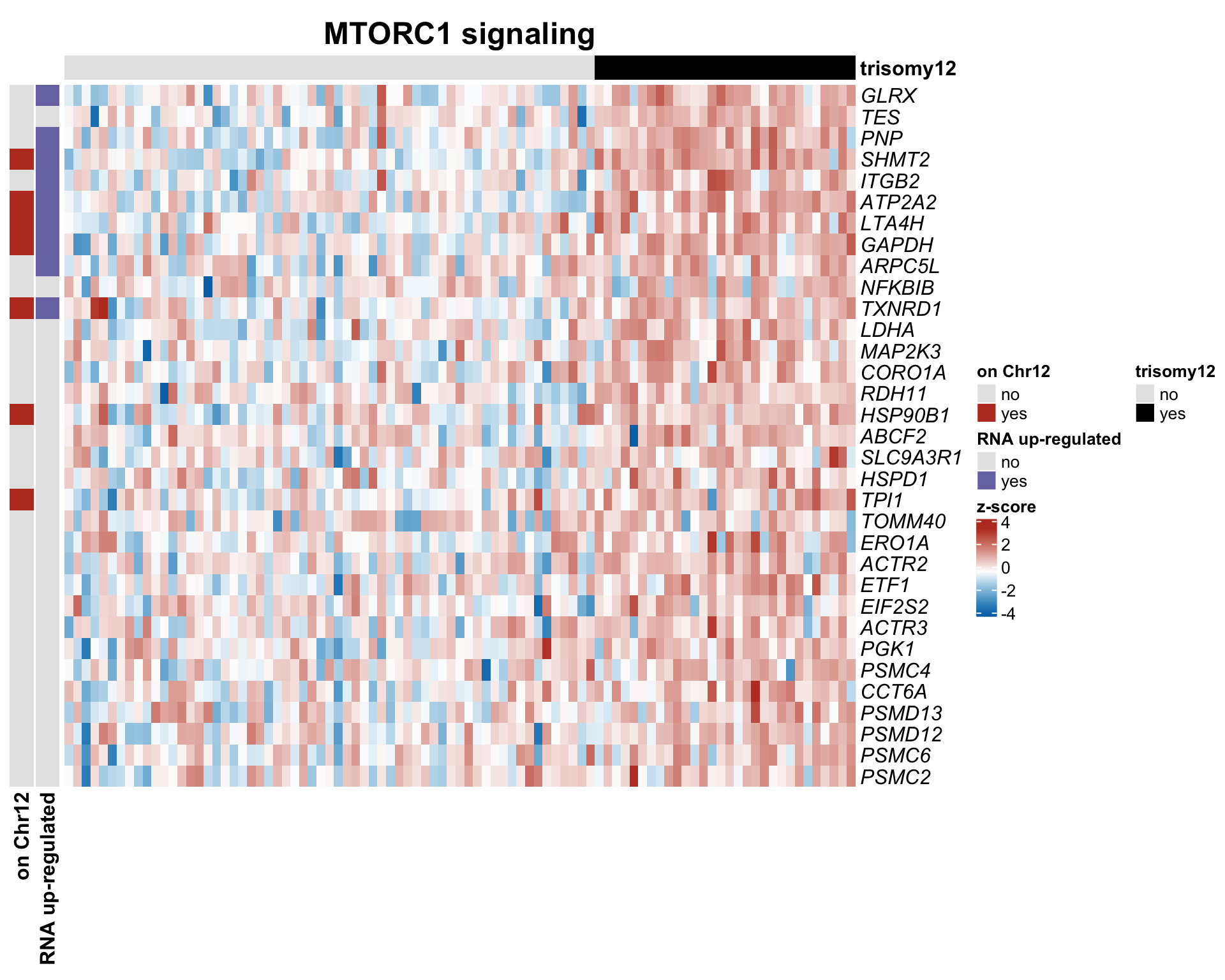
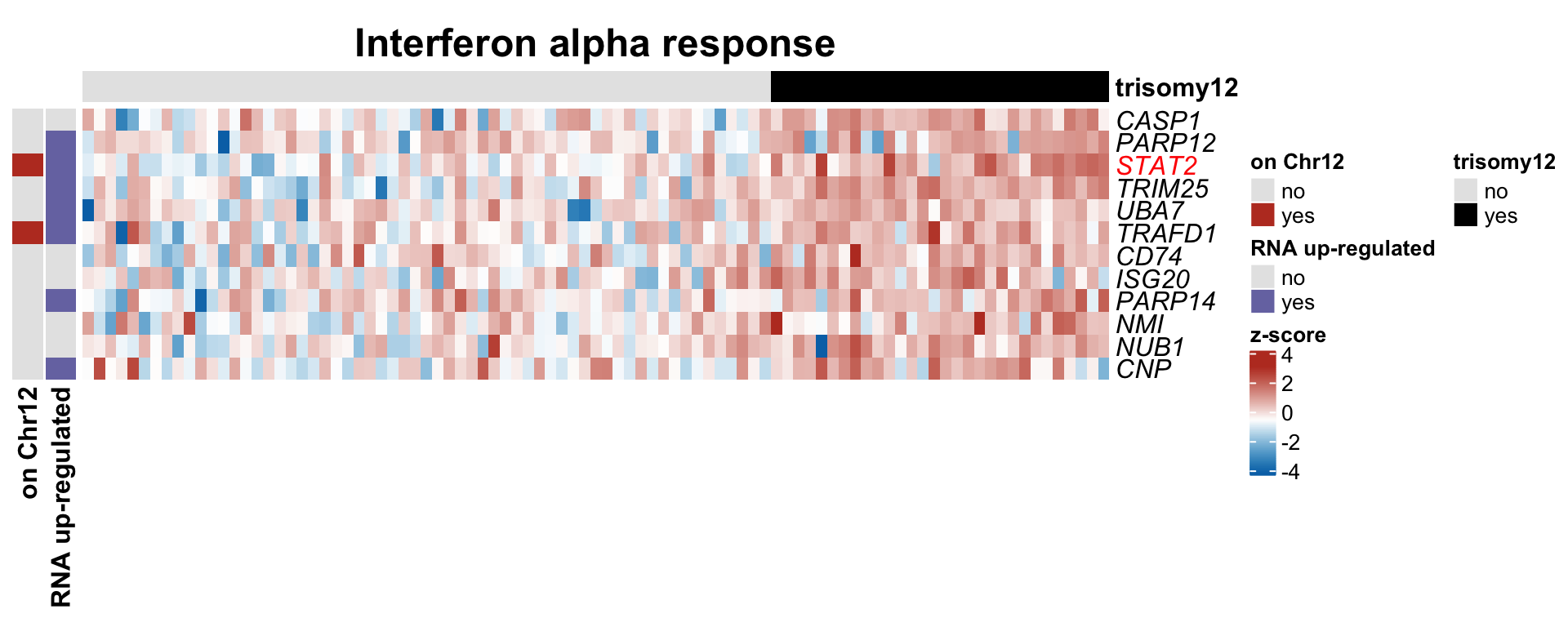
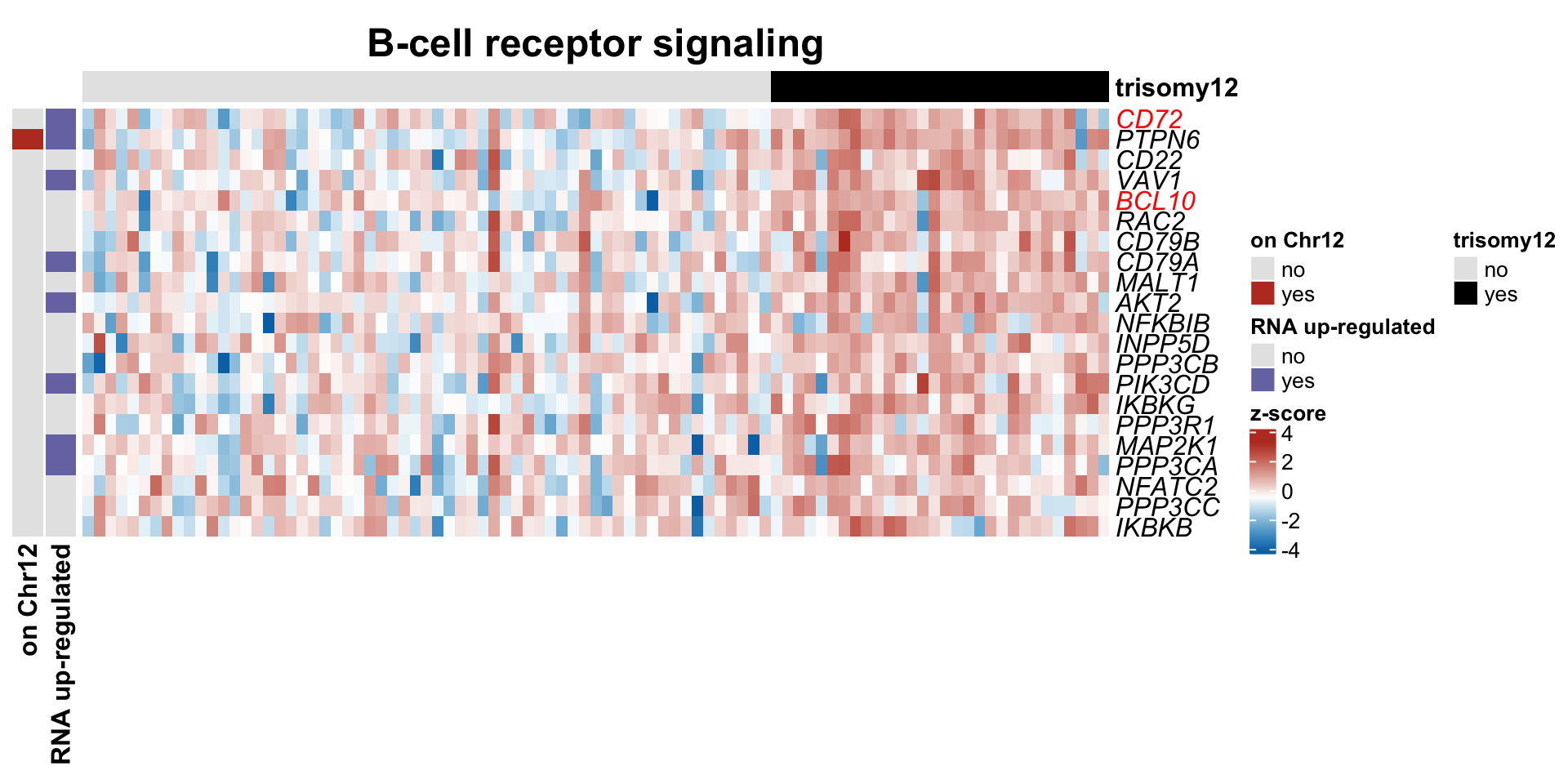
Section 3: Proteomic signatures of trisomy12
Junyan Lu
2021-02-16
Last updated: 2021-05-06
Checks: 5 2
Knit directory: CLLproteomics_publish_revision/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown is untracked by Git. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20200227) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
- unnamed-chunk-25
- unnamed-chunk-27
- unnamed-chunk-9
To ensure reproducibility of the results, delete the cache directory manuscript_S3_trisomy12_cache and re-run the analysis. To have workflowr automatically delete the cache directory prior to building the file, set delete_cache = TRUE when running wflow_build() or wflow_publish().
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 3fb50c5. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/.DS_Store
Ignored: analysis/.Rhistory
Ignored: analysis/manuscript_S1_Overview_cache/
Ignored: analysis/manuscript_S2_genomicAssociation_cache/
Ignored: analysis/manuscript_S3_trisomy12_cache/
Ignored: code/.DS_Store
Ignored: code/.Rhistory
Ignored: data/.DS_Store
Ignored: output/.DS_Store
Untracked files:
Untracked: analysis/.trisomy12_norm.pdf
Untracked: analysis/bufferComplexViolin.pdf
Untracked: analysis/buffer_Tri12vsTri19.pdf
Untracked: analysis/cohortComposition_all.pdf
Untracked: analysis/heatmap_tri12_circle.pdf
Untracked: analysis/manuscript_S1_Overview.Rmd
Untracked: analysis/manuscript_S2_genomicAssociation.Rmd
Untracked: analysis/manuscript_S3_trisomy12.Rmd
Untracked: analysis/manuscript_S4_trisomy19.Rmd
Untracked: analysis/manuscript_S5_IGHV.Rmd
Untracked: analysis/manuscript_S6_del11q.Rmd
Untracked: analysis/manuscript_S7_SF3B1.Rmd
Untracked: analysis/manuscript_S8_drugResponse_Outcomes.Rmd
Untracked: analysis/manuscript_S9_STAT2.Rmd
Untracked: analysis/plot_PC1_PC2.pdf
Untracked: analysis/timsTOF_validate.Rmd
Untracked: analysis/tri12_transEnrich.pdf
Untracked: analysis/trisomy12_chr_summary.pdf
Untracked: code/utils.R
Untracked: data/Annotation file March 2021.xlsx
Untracked: data/CAS9results.xlsx
Untracked: data/CNV_onChrom.RData
Untracked: data/ComplexParticipantsPubMedIdentifiers_human.txt
Untracked: data/Fig1A.png
Untracked: data/IGLV321_SupplementalTables_R2.xlsx
Untracked: data/MOFAout.RData
Untracked: data/MOFAout_atLeast3.RData
Untracked: data/STATexprPCR.xlsx
Untracked: data/Western_blot_results_20210309_short.csv
Untracked: data/Western_blot_results_separate_blots.xlsx
Untracked: data/allComplexes.txt
Untracked: data/ddsrna_enc.RData
Untracked: data/exprCNV_enc.RData
Untracked: data/geneAnno.RData
Untracked: data/gmts/
Untracked: data/ic50.RData
Untracked: data/mofaIn.RData
Untracked: data/mofaIn_atLeast3.RData
Untracked: data/patMeta_enc.RData
Untracked: data/pepCLL_lumos_enc.RData
Untracked: data/protMOFA.RData
Untracked: data/proteins_in_complexes
Untracked: data/proteomic_LUMOS_2pep_enc.RData
Untracked: data/proteomic_explore_enc.RData
Untracked: data/proteomic_independent_all_enc.RData
Untracked: data/proteomic_independent_enc.RData
Untracked: data/proteomic_timsTOF_enc.RData
Untracked: data/screenData_enc.RData
Untracked: data/setToPathway.txt
Untracked: data/survival_enc.RData
Untracked: output/MSH6_splicing.svg
Untracked: output/SUGP1_splicing.svg
Untracked: output/deResList.RData
Untracked: output/deResListBatch2.RData
Untracked: output/deResListRNA.RData
Untracked: output/deResListRNA_allGene.RData
Untracked: output/deResList_WBC.RData
Untracked: output/deResList_batch1.RData
Untracked: output/deResList_batch3.RData
Untracked: output/deResList_independent.RData
Untracked: output/deResList_timsTOF.RData
Untracked: output/dxdCLL.RData
Untracked: output/dxdCLL2.RData
Untracked: output/exprCNV.RData
Untracked: output/geneAnno.RData
Untracked: output/int_pairs.csv
Untracked: output/lassoResults_CPS.RData
Untracked: output/resOutcome_batch1.RData
Untracked: output/resOutcome_batch13.RData
Untracked: output/resOutcome_batch2.RData
Untracked: output/resOutcome_batch3.RData
Unstaged changes:
Modified: analysis/_site.yml
Deleted: analysis/analysisSF3B1.Rmd
Deleted: analysis/comparePlatforms.Rmd
Deleted: analysis/compareProteomicsRNAseq.Rmd
Deleted: analysis/correlateCLLPD.Rmd
Deleted: analysis/correlateGenomic.Rmd
Deleted: analysis/correlateGenomic_removePC.Rmd
Deleted: analysis/correlateMIR.Rmd
Deleted: analysis/correlateMethylationCluster.Rmd
Modified: analysis/index.Rmd
Deleted: analysis/predictOutcome.Rmd
Deleted: analysis/processProteomics_LUMOS.Rmd
Deleted: analysis/processProteomics_timsTOF.Rmd
Deleted: analysis/qualityControl_LUMOS.Rmd
Deleted: analysis/qualityControl_timsTOF.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
There are no past versions. Publish this analysis with wflow_publish() to start tracking its development.
#Load packages and datasets
Overview of differentially expressed proteins realted to trisomy12
A table of associations with 5% FDR
resList <- filter(resList, Gene == "trisomy12") %>%
#mutate(adj.P.Val = adj.P.global) %>% #use global corrected P-value
mutate(Chr = rowData(protCLL[id,])$chromosome_name)
resList %>% filter(adj.P.Val <= 0.05) %>%
select(name, Chr,logFC, P.Value, adj.P.Val) %>%
mutate_if(is.numeric, formatC, digits=2) %>%
DT::datatable()Heatmap of differentially expressed proteins
proList <- filter(resList, !is.na(name), adj.P.Val < 0.01) %>% distinct(name, .keep_all = TRUE) %>% pull(id)
plotMat <- assays(protCLL)[["QRILC_combat"]][proList,]
rownames(plotMat) <- rowData(protCLL[proList,])$hgnc_symbol
colAnno <- filter(patMeta, Patient.ID %in% colnames(protCLL)) %>%
select(Patient.ID, trisomy12, IGHV.status) %>%
data.frame() %>% column_to_rownames("Patient.ID")
colAnno$trisomy12 <- ifelse(colAnno$trisomy12 %in% 1, "yes","no")
rowAnno <- rowData(protCLL)[proList,c("chromosome_name","hgnc_symbol"),drop=FALSE] %>%
data.frame(stringsAsFactors = FALSE) %>%
mutate(onChr12 = ifelse(chromosome_name == "12","yes","no")) %>%
select(hgnc_symbol, onChr12) %>% data.frame() %>% remove_rownames() %>% column_to_rownames("hgnc_symbol")
plotMat <- jyluMisc::mscale(plotMat, censor = 5)
annoCol <- list(trisomy12 = c(yes = "black",no = "grey80"),
IGHV.status = c(M = colList[4], U = colList[3]),
onChr12 = c(yes = colList[1],no = "white"))
pheatmap::pheatmap(plotMat, annotation_col = colAnno, scale = "none",
annotation_row = rowAnno,
clustering_method = "complete", clustering_distance_cols = "euclidean",
color = colorRampPalette(c(colList[2],"white",colList[1]))(100),
breaks = seq(-5,5, length.out = 101), annotation_colors = annoCol,
show_rownames = FALSE, show_colnames = FALSE,
treeheight_row = 0)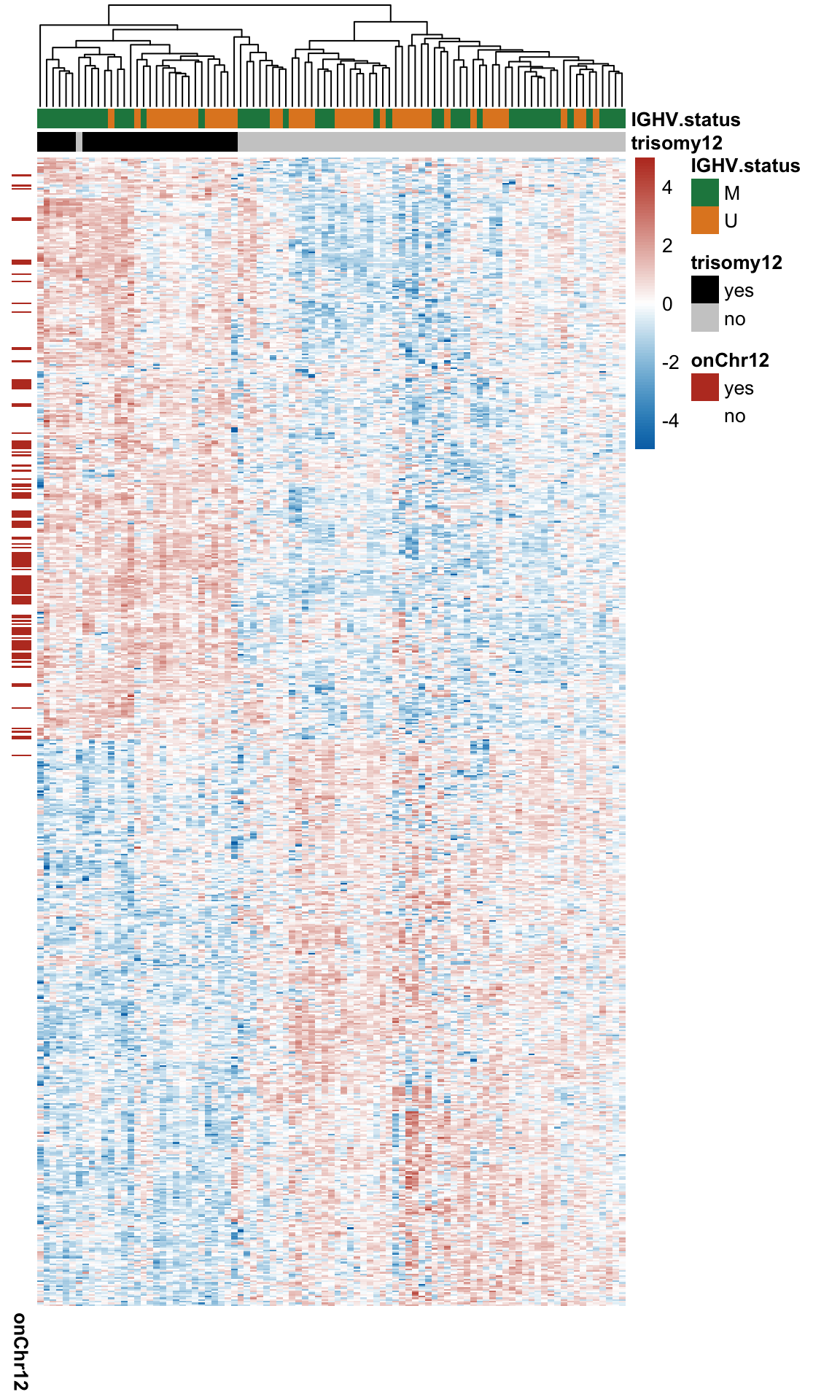
Summary of the chromosome distribution of genes that encode those DE proteins
plotTab <- filter(resList, adj.P.Val <=0.05) %>% mutate(change = ifelse(logFC>0,"Up regulated","Down regulated"),
chromosome = ifelse(Chr %in% "12","chr12","other")) %>%
group_by(change, chromosome) %>% summarise(n = length(id))
sigNumPlot <- ggplot(plotTab, aes(x=change, y=n, fill = chromosome)) +
geom_bar(stat = "identity", width = 0.8,
position = position_dodge2(width = 6),
col = "black") +
geom_text(aes(label=n),
position = position_dodge(width = 0.9),
size=4, hjust=-0.1) +
scale_fill_manual(name = "", labels = c("chr12","other"), values = colList) +
coord_flip(ylim = c(0,700), expand = FALSE) + xlab("") + ylab("Number of associations (5% FDR)") + theme_half +
theme(legend.text = element_text(size=12))
sigNumPlot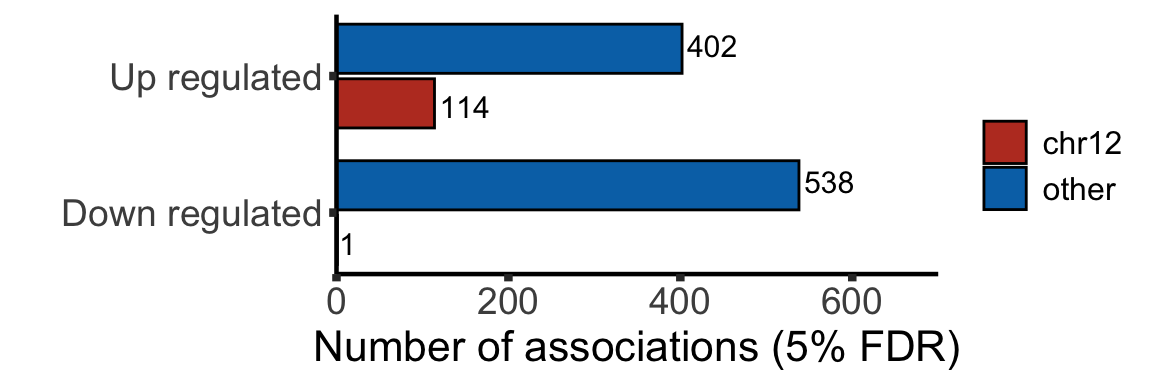
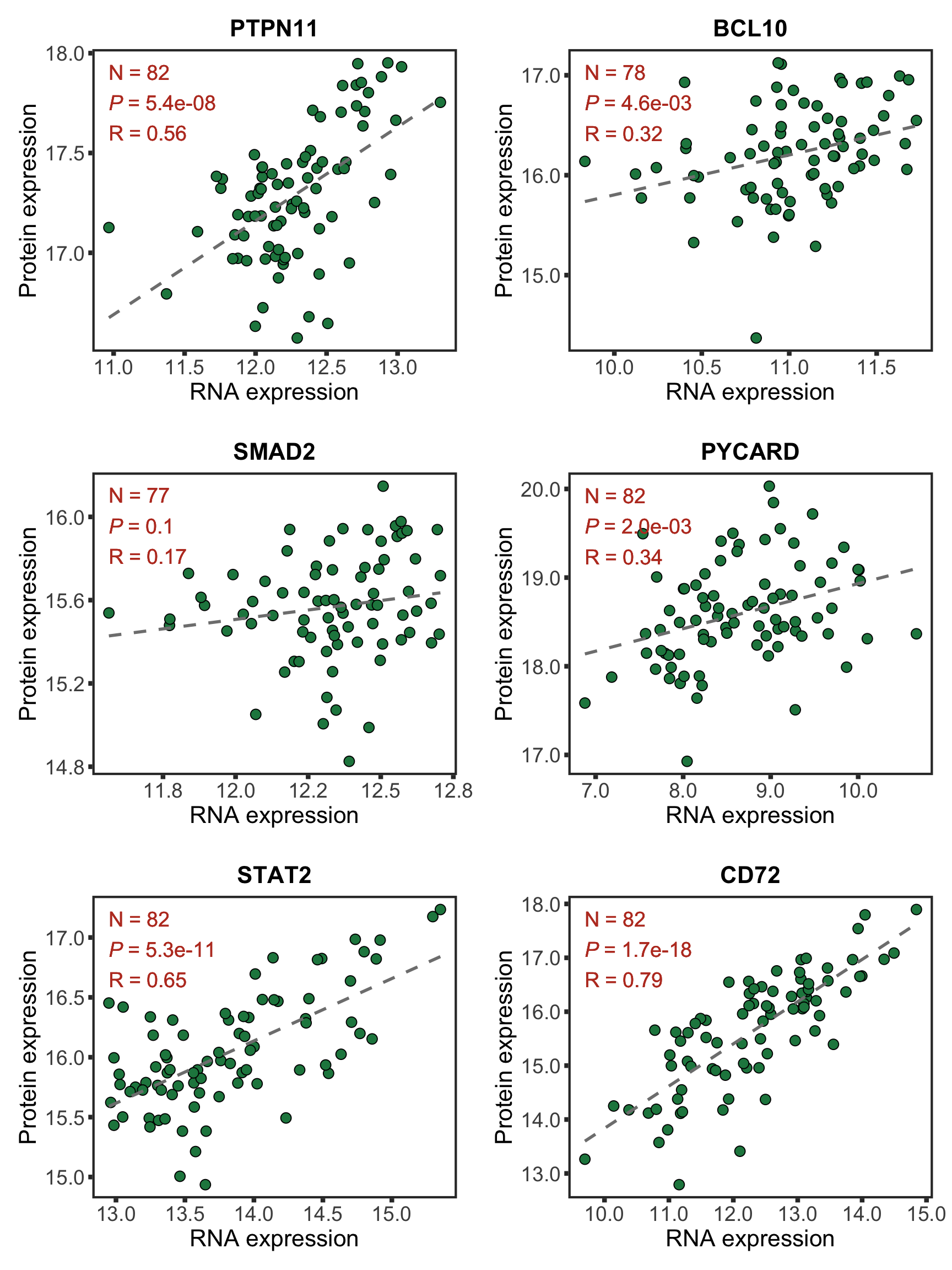
Volcano plot for differentially expressed proteins.
plotTab <- resList
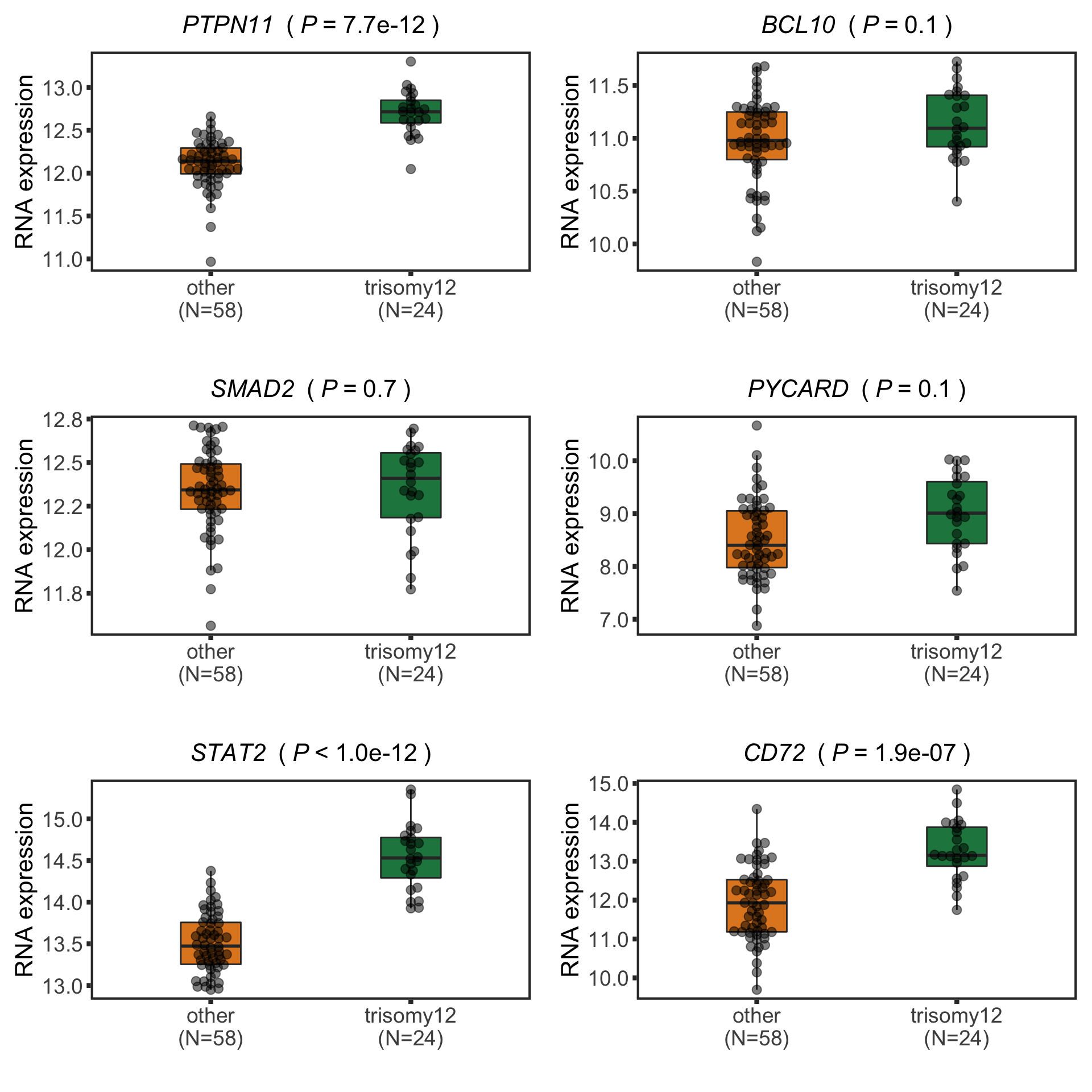
nameList <- c("PTPN11", "BCL10", "SMAD2", "PYCARD", "STAT2", "CD72")
tri12Vocano <- plotVolcano(plotTab, fdrCut =0.05, x_lab=bquote("log"[2]*"(fold change)"), posCol = colList[1], negCol = colList[2],
plotTitle = "trisomy12 (present versus absent)", ifLabel = TRUE, labelList = nameList)
tri12Vocano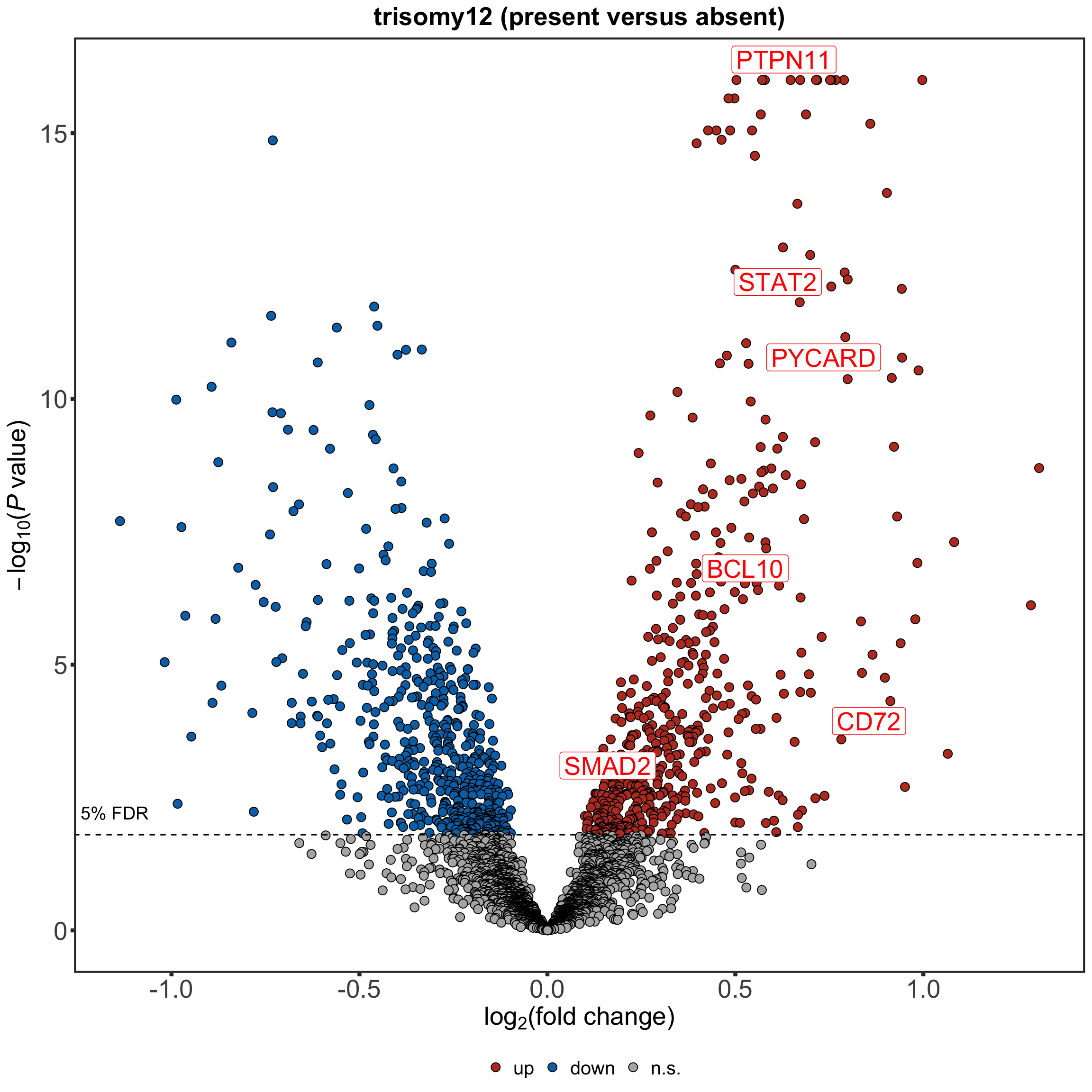 The proteins discussed in the manuscript are labelled.
The proteins discussed in the manuscript are labelled.
Boxplot plot of selected genes
protTab <- sumToTidy(protCLL, rowID = "uniprotID", colID = "patID") %>%
mutate(count = count_combat)
plotTab <- protTab %>% filter(hgnc_symbol %in% nameList) %>%
mutate(trisomy12 = patMeta[match(patID, patMeta$Patient.ID),]$trisomy12) %>%
mutate(status = ifelse(trisomy12 %in% 1,"trisomy12","other"),
name = hgnc_symbol) %>%
mutate(status = factor(status, levels = c("other","trisomy12")))
pList <- plotBox(plotTab, pValTabel = resList, y_lab = "Protein expression")
protBoxplot <- cowplot::plot_grid(plotlist= pList, ncol=3)
protBoxplot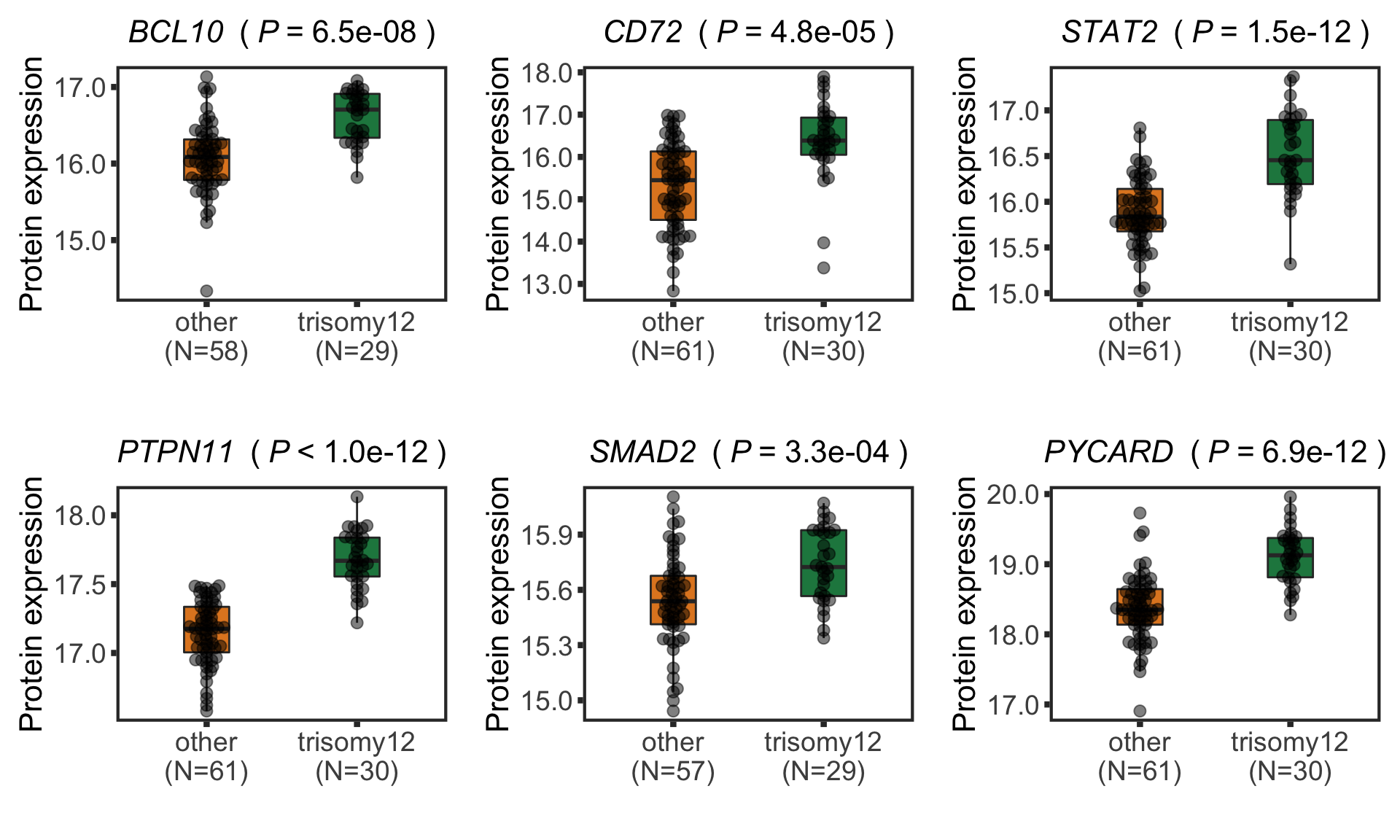
#ggsave("trisomy12_selected_protein.pdf", height = 6, width = 10)Protein complex analysis
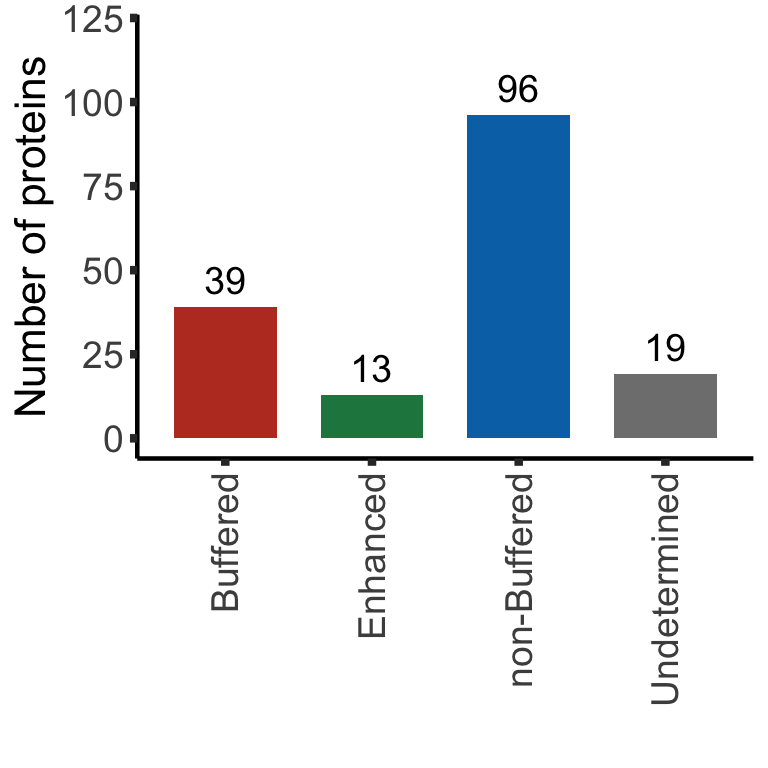
Data preprocessing
Read stable protein complex pairs
int_pairs <- read_csv2("../output/int_pairs.csv")Whether complex formation affects protein buffering?
Compare log2 fold changes difference
testTab <- bufferTab %>% mutate(inComplex = ifelse(uniprotID %in% c(int_pairs$ProtA,int_pairs$ProtB),
"complex in","complex out")) %>%
group_by(inComplex) %>% mutate(n = length(name)) %>%
mutate(xLabel = sprintf("%s\n(N=%s)",inComplex,n))
tRes <- t.test(diffFC~inComplex, testTab)
pVal <- formatC(tRes$p.value, digits = 1, format="e")
pLab <- bquote(italic("P")~"="~.(pVal))
ggplot(testTab, aes(x=xLabel, y=diffFC)) +
geom_violin(aes(fill = xLabel), trim=FALSE) +
stat_summary(fun.data="mean_sdl", mult=1,
geom="errorbar", width=0.05) +
stat_summary(fun.y=mean, geom="point") +
scale_fill_manual(values = colList[4:5]) +
theme_full +
xlab("") + ylab("log2(RNA fold change) - log2(protein fold change)") +
annotate("text", label = pLab , x=Inf, y=Inf, hjust=1.2, vjust=2,size=6) +
theme(legend.position = "none",
axis.text.y = element_text(size=15),
axis.text.x = element_text(size=18),
axis.title = element_text(size=15)) 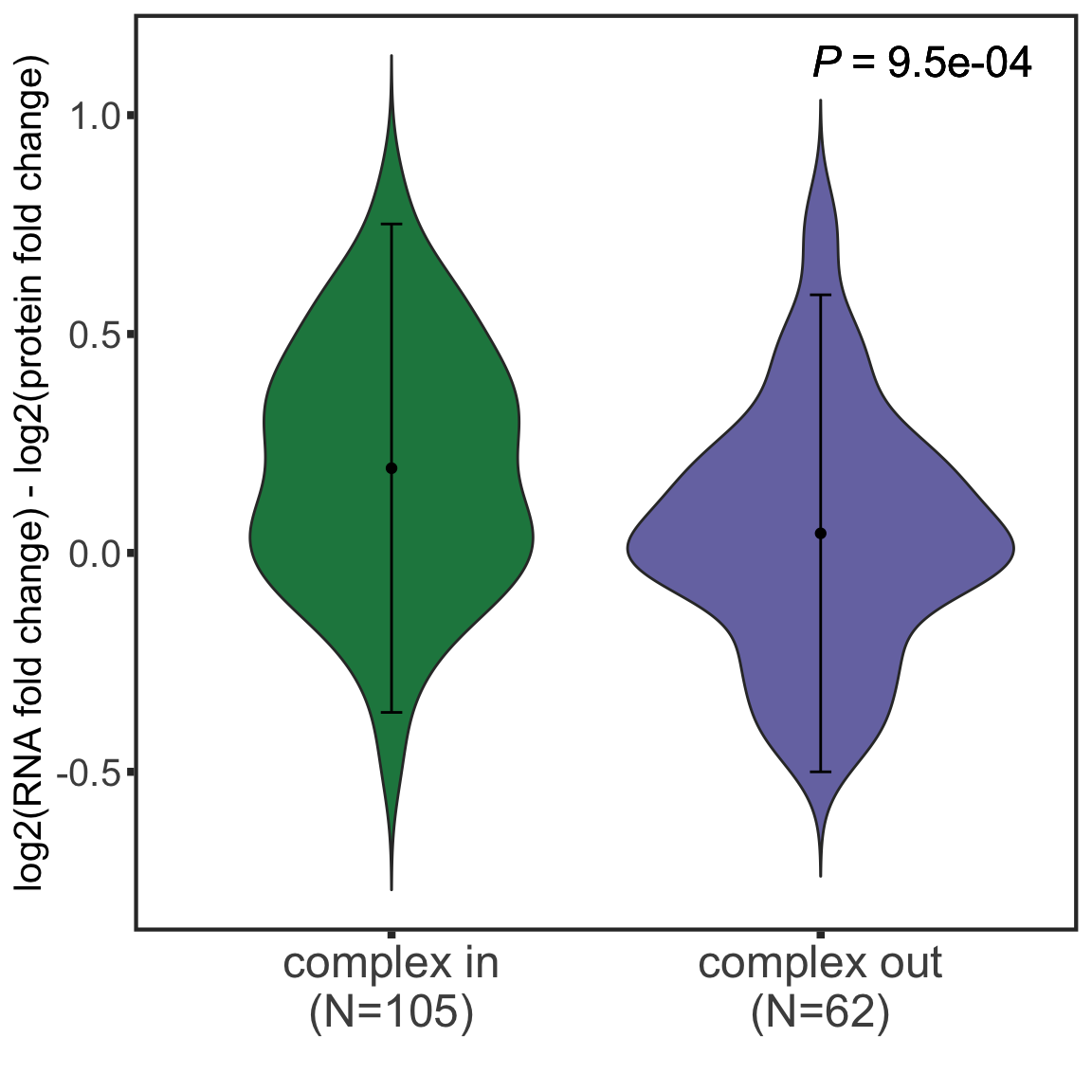
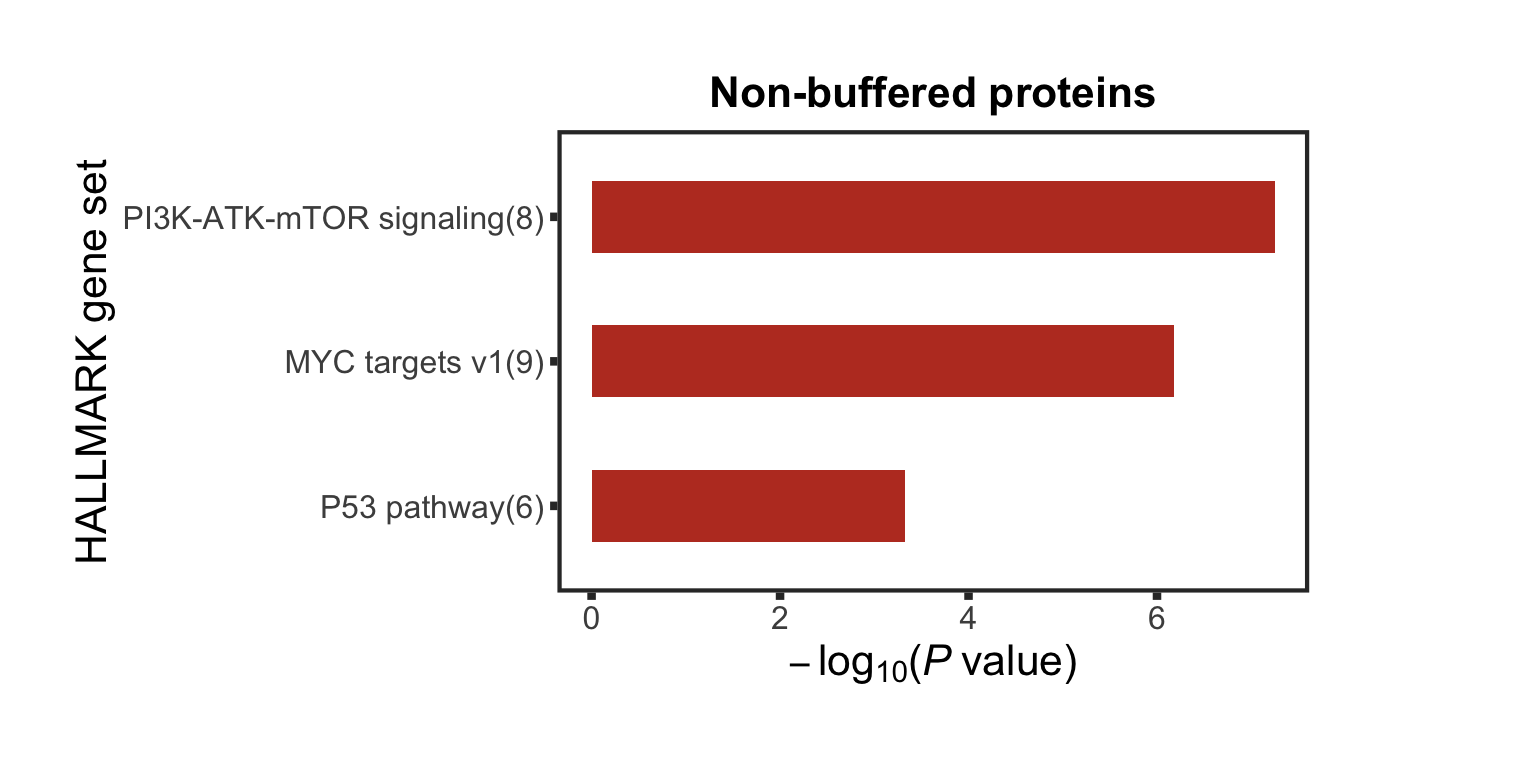
#ggsave("bufferComplexViolin.pdf", height = 6, width = 4.5)Construct protein-protein interaction network by connecting chr12 proteins and non-chr12 protein
comTab <- int_pairs %>% select(ProtA, ProtB, database) %>%
mutate(chrA = rowData(protCLL[ProtA,])$chromosome_name,
chrB = rowData(protCLL[ProtB,])$chromosome_name) %>%
filter(!is.na(chrA), !is.na(chrB)) %>%
filter((chrA == "12" & chrB != "12") | (chrA !="12" & chrB == "12")) %>%
mutate(source = ifelse(chrA == 12, ProtA, ProtB),
target = ifelse(chrA == 12, ProtB, ProtA)) %>%
select(source, target, database)fdrCut <- 0.05
resTab <- select(allRes, name, uniprotID, chrom, padj, padj.rna, log2FC,log2FC.rna) %>%
mutate(sigProt = padj <= fdrCut,
sigRna = padj.rna <=fdrCut,
upProt = sigProt & log2FC > 0,
upRna = sigRna & log2FC.rna > 0)
comTab <- comTab %>%
left_join(resTab, by = c(source = "uniprotID")) %>%
left_join(resTab, by = c(target = "uniprotID")) %>%
rename_all(funs(str_replace(., "x", "source"))) %>%
rename_all(funs(str_replace(., "y", "target"))) comTab.filter <- filter(comTab, upRna.source, upProt.source, upProt.target)#get node list
allNodes <- union(comTab.filter$name.source, comTab.filter$name.target)
nodeList <- data.frame(id = seq(length(allNodes))-1, name = allNodes, stringsAsFactors = FALSE) %>%
mutate(chromosome = ifelse(rowData(protCLL[match(name, rowData(protCLL)$hgnc_symbol),])$chromosome_name %in% "12",
"chr12","other"))
#get edge list
edgeList <- select(comTab.filter, name.source, name.target, database, upRna.target) %>%
dplyr::rename(Source = name.source, Target = name.target) %>%
mutate(Source = nodeList[match(Source,nodeList$name),]$id,
Target = nodeList[match(Target, nodeList$name),]$id,
sigRNA = ifelse(upRna.target,"yes","no")) %>%
data.frame(stringsAsFactors = FALSE)
net <- graph_from_data_frame(vertices = nodeList, d=edgeList, directed = FALSE)tidyNet <- as_tbl_graph(net)
complexNet <- ggraph(tidyNet, layout = "igraph", algorithm = "nicely") +
geom_edge_link(color = colList[3], width=1) +
geom_node_point(aes(color =chromosome, shape = chromosome), size=6) +
geom_node_text(aes(label = name), repel = TRUE, size=6) +
#scale_edge_linetype_manual(values = c(no = "dotted", yes = "solid"), name = "RNA up-regulated")+
scale_color_manual(values = c(chr12 = colList[1],other = colList[2])) +
scale_edge_color_brewer(palette = "Set2") +
theme_graph(base_family = "sans") + theme(legend.position = "bottom")
complexNet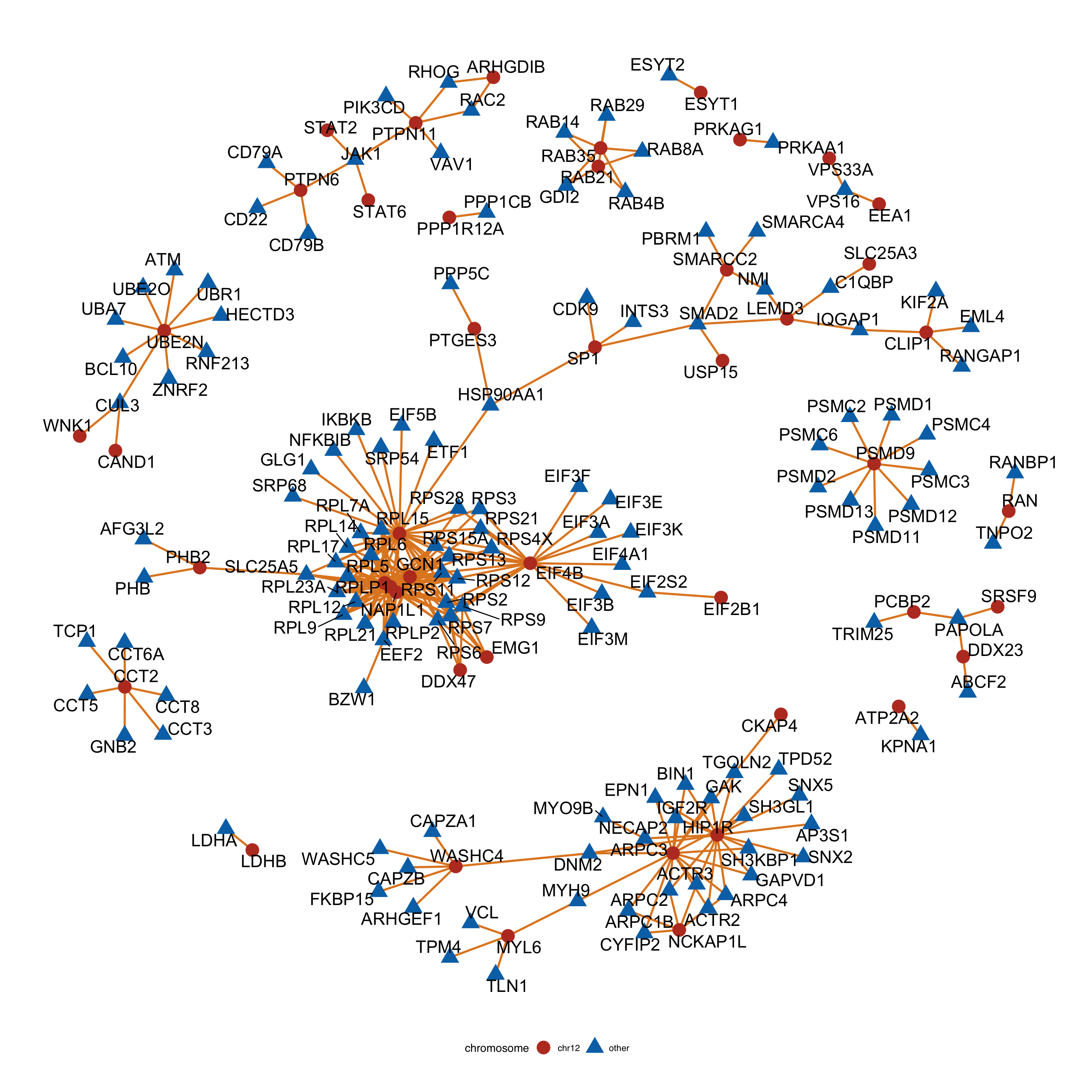
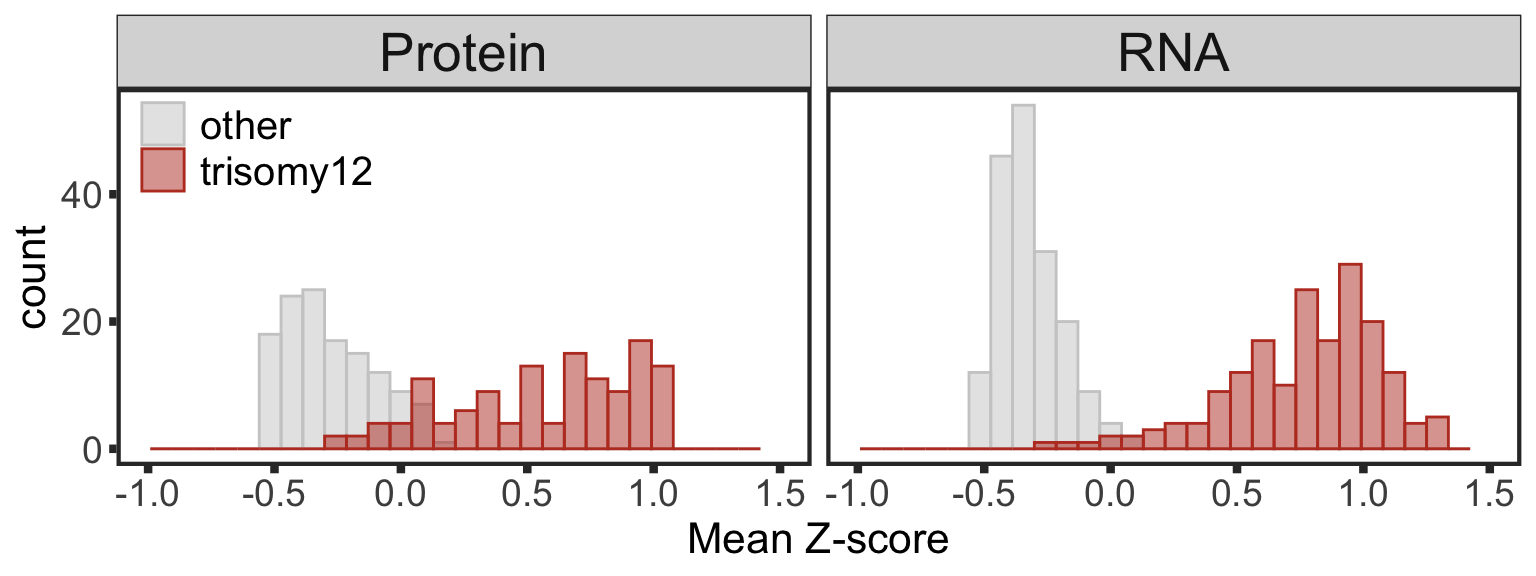
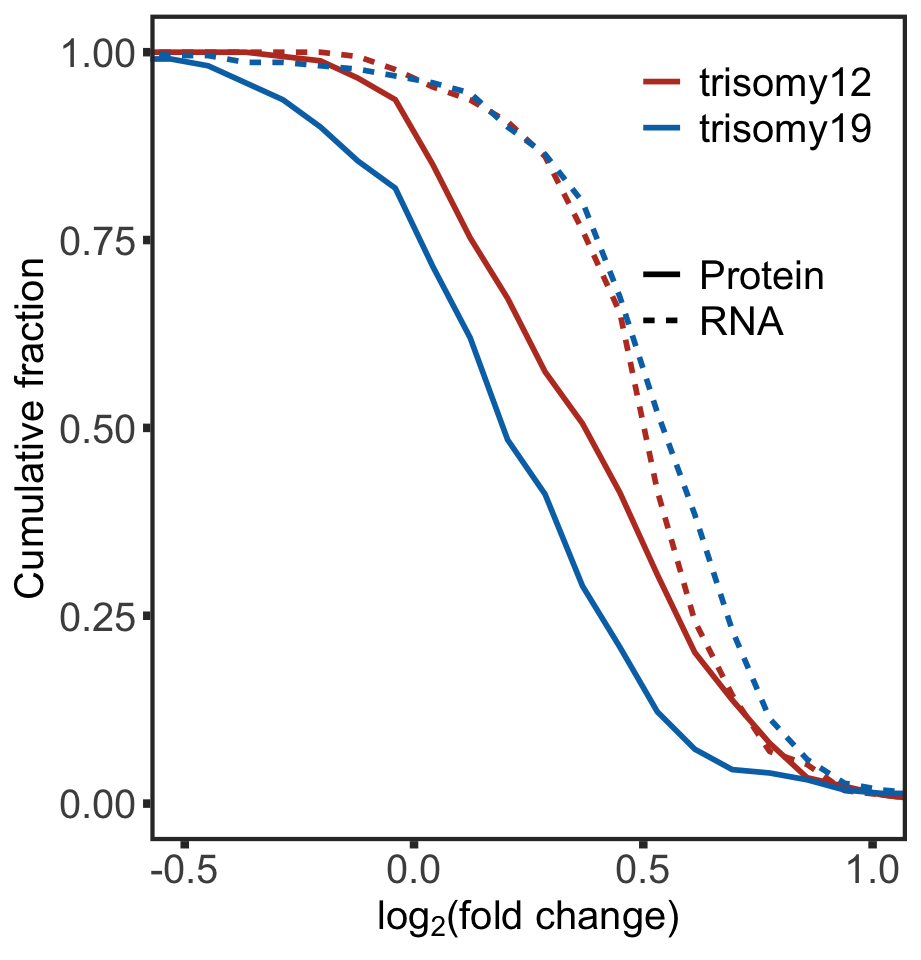
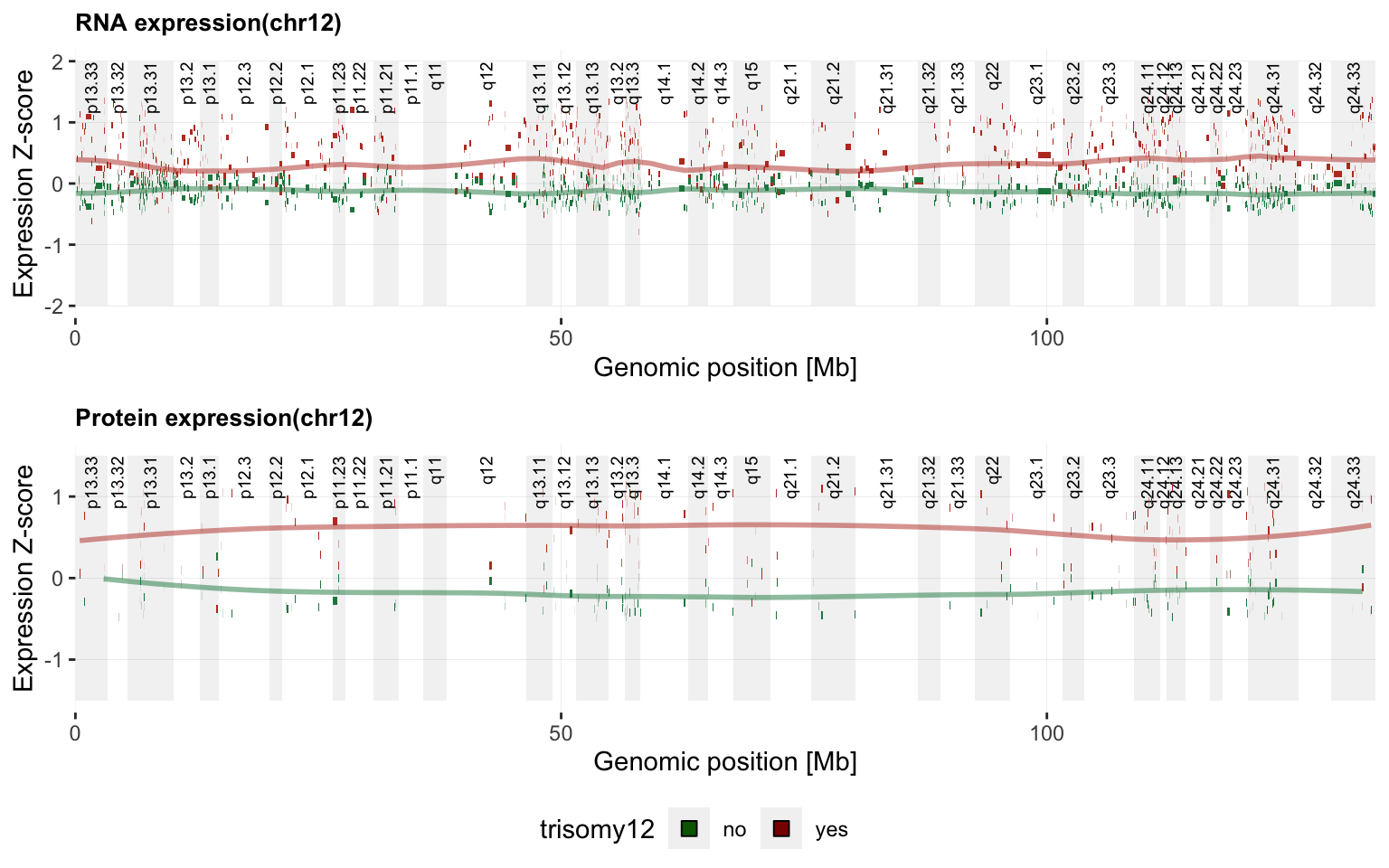
#ggsave("trisomy12Complex.pdf", height = 15, width = 15)Associate gene expression, protein expression and pathways on Chr12 and Chr19
load("../output/deResListRNA_allGene.RData")
load("../output/deResList.RData")
rnaRes <- resListRNA_allGene %>% filter(Gene == "trisomy12") %>%
mutate(Chr = rowData(dds[id,])$chromosome) %>%
filter(Chr == "12", adj.P.Val <= 0.05, t > 0) %>%
distinct(name)
protRes <- resList %>% filter(Gene == "trisomy12") %>%
mutate(Chr = rowData(protCLL[id,])$chromosome_name) %>%
filter(Chr == "12", adj.P.Val <=0.05, t >0) %>%
distinct(name)Associations between PI3K/Akt/mTOR inhibitor responses and trisomy12
Prepare table
load("../data/screenData_enc.RData")
screenData <- screenData %>% filter(patientID %in% colnames(protCLL),
Drug %in% c("Duvelisib","MK-2206","Rapamycin")) %>%
group_by(patientID, Drug) %>% summarise(viab = mean(normVal.cor_auc)) %>%
mutate(trisomy12 = patMeta[match(patientID, patMeta$Patient.ID),]$trisomy12,
IGHV = patMeta[match(patientID, patMeta$Patient.ID),]$IGHV.status) %>%
mutate(status = ifelse(trisomy12==1,"trisomy12","other"),
IGHV = ifelse(IGHV=="M","M-CLL","U-CLL"),
Drug = as.character(Drug))Sample size
table(distinct(screenData, patientID, trisomy12)$trisomy12)
0 1
59 23 T-test
All samples
tRes <- group_by(screenData, Drug) %>% nest() %>%
mutate(m = map(data, ~t.test(viab~trisomy12,.))) %>%
mutate(res = map(m, broom::tidy)) %>%
unnest(res) %>%
select(Drug, estimate, p.value)
tRes# A tibble: 3 x 3
# Groups: Drug [3]
Drug estimate p.value
<chr> <dbl> <dbl>
1 Duvelisib 0.0787 0.0117
2 MK-2206 0.0324 0.00631
3 Rapamycin 0.0568 0.0139 IGHV stratified
tRes.ighv <- group_by(screenData, Drug, IGHV) %>% nest() %>%
mutate(m = map(data, ~t.test(viab~trisomy12,.))) %>%
mutate(res = map(m, broom::tidy)) %>%
unnest(res) %>%
select(Drug,IGHV, estimate, p.value)
tRes.ighv# A tibble: 6 x 4
# Groups: Drug, IGHV [6]
Drug IGHV estimate p.value
<chr> <chr> <dbl> <dbl>
1 Duvelisib M-CLL 0.0438 0.340
2 MK-2206 M-CLL 0.0111 0.406
3 Rapamycin M-CLL 0.0507 0.217
4 Duvelisib U-CLL 0.103 0.000474
5 MK-2206 U-CLL 0.0517 0.00422
6 Rapamycin U-CLL 0.0637 0.0122 Visualization
All samples
Function for plot drug effect as boxplots
plotDrugBox <- function(screenData, tRes, y_lab = "Viability") {
ymax <- max(screenData$viab)
ymin <- min(screenData$viab)
plotList <- lapply(unique(screenData$Drug), function(n) {
eachTab <- filter(screenData, Drug == n) %>%
group_by(status) %>% mutate(n=n()) %>% ungroup() %>%
mutate(group = sprintf("%s\n(N=%s)",status,n)) %>%
arrange(status) %>% mutate(group = factor(group, levels = unique(group)))
pval <- formatNum(filter(tRes, Drug == n)$p.value, digits = 1, format="e")
annoText <- bquote(.(n)~" ("~italic("P")~"="~.(pval)~")")
ggplot(eachTab, aes(x=group, y = viab)) +
geom_beeswarm(aes(col=IGHV), size =2.5,cex = 2, alpha=0.8) +
geom_boxplot(fill = NA, width=0.3, outlier.shape = NA) +
ggtitle(annoText)+
#ggtitle(sprintf("%s (p = %s)",geneName, formatNum(pval, digits = 1, format = "e"))) +
ylab(y_lab) + xlab("") +
scale_color_manual(values = colList[2:3]) +
scale_y_continuous(limits=c(ymin,ymax),labels = scales::number_format(accuracy = 0.1))+
theme_full +
theme(legend.position = "bottom",
plot.title = element_text(hjust = 0.5),
plot.margin = margin(0,20,0,20))
})
return(plotList)
}All samples
pList <- plotDrugBox(screenData, tRes)
plot_grid(plotlist = pList, ncol=3)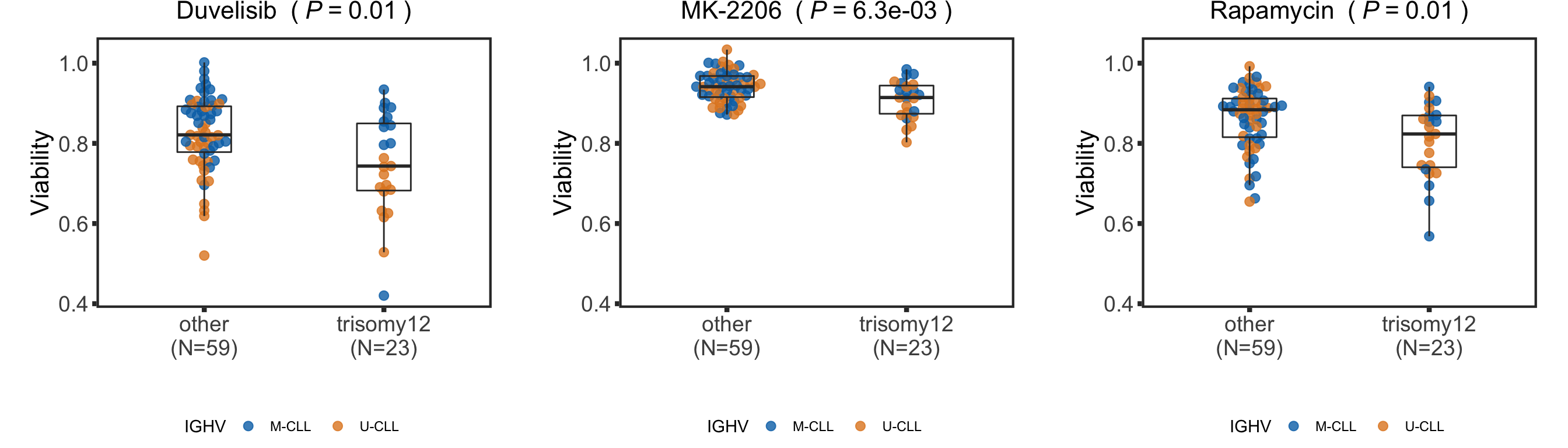
IGHV stratified
Function for plot drug effect as boxplots
plotDrugBoxIGHV <- function(screenData, tRes.ighv, y_lab = "Viability") {
screenData <- mutate(screenData, drugIGHV =paste0(Drug,"_",IGHV))
tRes.ighv <- mutate(tRes.ighv, drugIGHV = paste0(Drug, "_",IGHV))
ymax <- max(screenData$viab)
ymin <- min(screenData$viab)
plotList <- lapply(unique(screenData$drugIGHV), function(n) {
eachTab <- filter(screenData, drugIGHV == n) %>%
group_by(status) %>% mutate(n=n()) %>% ungroup() %>%
mutate(group = sprintf("%s\n(N=%s)",status,n)) %>%
arrange(status) %>% mutate(group = factor(group, levels = unique(group)))
drug <- unique(eachTab$Drug)
ighv <- unique(eachTab$IGHV)
pval <- formatNum(filter(tRes.ighv, drugIGHV == n)$p.value, digits = 1, format="e")
annoText <- bquote(.(drug)~" ("~italic("P")~"="~.(pval)~","~.(ighv)~")")
ggplot(eachTab, aes(x=group, y = viab)) +
geom_beeswarm(aes(col=IGHV), size =2.5,cex = 2, alpha=0.8) +
geom_boxplot(fill = NA, width=0.3, outlier.shape = NA) +
ggtitle(annoText)+
#ggtitle(sprintf("%s (p = %s)",geneName, formatNum(pval, digits = 1, format = "e"))) +
ylab(y_lab) + xlab("") +
scale_color_manual(values = c(`M-CLL` = colList[2], `U-CLL` = colList[3])) +
scale_y_continuous(limits=c(ymin,ymax), labels = scales::number_format(accuracy = 0.1))+
theme_full +
theme(legend.position = "none",
plot.title = element_text(hjust = 0.5),
plot.margin = margin(20,20,20,20))
})
return(plotList)
}pList <- plotDrugBoxIGHV(screenData, tRes.ighv)
plot_grid(plotlist = pList, ncol=3)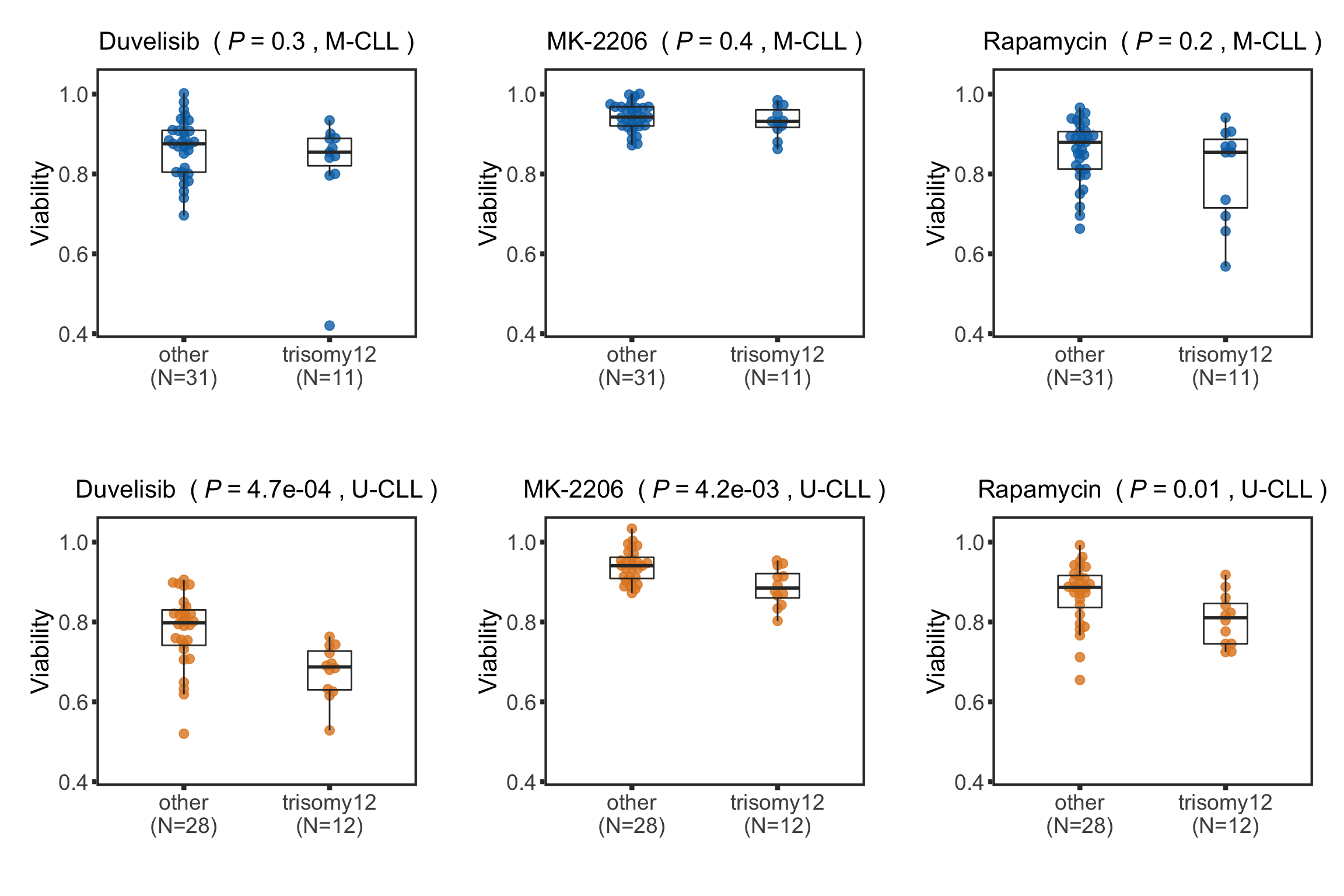
sessionInfo()R version 4.0.2 (2020-06-22)
Platform: x86_64-apple-darwin17.0 (64-bit)
Running under: macOS 10.16
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.0/Resources/lib/libRblas.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.0/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] grid parallel stats4 stats graphics grDevices utils
[8] datasets methods base
other attached packages:
[1] circlize_0.4.12 piano_2.4.0
[3] latex2exp_0.4.0 forcats_0.5.1
[5] stringr_1.4.0 dplyr_1.0.5
[7] purrr_0.3.4 readr_1.4.0
[9] tidyr_1.1.3 tibble_3.1.0
[11] tidyverse_1.3.0 ggbeeswarm_0.6.0
[13] ComplexHeatmap_2.4.3 pheatmap_1.0.12
[15] proDA_1.2.0 ggraph_2.0.5
[17] ggplot2_3.3.3 igraph_1.2.6
[19] cowplot_1.1.1 tidygraph_1.2.0
[21] DESeq2_1.28.1 SummarizedExperiment_1.18.2
[23] DelayedArray_0.14.1 matrixStats_0.58.0
[25] Biobase_2.48.0 GenomicRanges_1.40.0
[27] GenomeInfoDb_1.24.2 IRanges_2.22.2
[29] S4Vectors_0.26.1 BiocGenerics_0.34.0
[31] limma_3.44.3
loaded via a namespace (and not attached):
[1] rappdirs_0.3.3 exactRankTests_0.8-31 bit64_4.0.5
[4] knitr_1.31 multcomp_1.4-16 data.table_1.14.0
[7] rpart_4.1-15 RCurl_1.98-1.2 generics_0.1.0
[10] TH.data_1.0-10 RSQLite_2.2.3 bit_4.0.4
[13] xml2_1.3.2 lubridate_1.7.10 httpuv_1.5.5
[16] assertthat_0.2.1 viridis_0.5.1 xfun_0.21
[19] hms_1.0.0 jquerylib_0.1.3 evaluate_0.14
[22] promises_1.2.0.1 fansi_0.4.2 progress_1.2.2
[25] caTools_1.18.1 dbplyr_2.1.0 readxl_1.3.1
[28] km.ci_0.5-2 DBI_1.1.1 geneplotter_1.66.0
[31] htmlwidgets_1.5.3 ellipsis_0.3.1 jyluMisc_0.1.5
[34] crosstalk_1.1.1 ggpubr_0.4.0 backports_1.2.1
[37] annotate_1.66.0 vctrs_0.3.6 abind_1.4-5
[40] cachem_1.0.4 withr_2.4.1 ggforce_0.3.3
[43] checkmate_2.0.0 prettyunits_1.1.1 cluster_2.1.1
[46] crayon_1.4.1 drc_3.0-1 relations_0.6-9
[49] genefilter_1.70.0 pkgconfig_2.0.3 slam_0.1-48
[52] labeling_0.4.2 tweenr_1.0.1 nlme_3.1-152
[55] vipor_0.4.5 nnet_7.3-15 rlang_0.4.10
[58] lifecycle_1.0.0 sandwich_3.0-0 BiocFileCache_1.12.1
[61] modelr_0.1.8 cellranger_1.1.0 rprojroot_2.0.2
[64] polyclip_1.10-0 Matrix_1.3-2 KMsurv_0.1-5
[67] carData_3.0-4 zoo_1.8-9 reprex_1.0.0
[70] base64enc_0.1-3 beeswarm_0.3.1 GlobalOptions_0.1.2
[73] png_0.1-7 viridisLite_0.3.0 rjson_0.2.20
[76] bitops_1.0-6 shinydashboard_0.7.1 KernSmooth_2.23-18
[79] visNetwork_2.0.9 blob_1.2.1 workflowr_1.6.2
[82] shape_1.4.5 maxstat_0.7-25 jpeg_0.1-8.1
[85] rstatix_0.7.0 ggsignif_0.6.1 scales_1.1.1
[88] memoise_2.0.0 magrittr_2.0.1 gplots_3.1.1
[91] zlibbioc_1.34.0 compiler_4.0.2 RColorBrewer_1.1-2
[94] plotrix_3.8-1 clue_0.3-58 cli_2.3.1
[97] XVector_0.28.0 htmlTable_2.1.0 Formula_1.2-4
[100] MASS_7.3-53.1 mgcv_1.8-34 tidyselect_1.1.0
[103] stringi_1.5.3 highr_0.8 yaml_2.2.1
[106] askpass_1.1 locfit_1.5-9.4 latticeExtra_0.6-29
[109] ggrepel_0.9.1 survMisc_0.5.5 sass_0.3.1
[112] fastmatch_1.1-0 tools_4.0.2 rio_0.5.26
[115] rstudioapi_0.13 foreign_0.8-81 git2r_0.28.0
[118] gridExtra_2.3 farver_2.1.0 digest_0.6.27
[121] shiny_1.6.0 Rcpp_1.0.6 car_3.0-10
[124] broom_0.7.5 later_1.1.0.1 httr_1.4.2
[127] survminer_0.4.9 AnnotationDbi_1.50.3 colorspace_2.0-0
[130] rvest_1.0.0 XML_3.99-0.5 fs_1.5.0
[133] splines_4.0.2 graphlayouts_0.7.1 xtable_1.8-4
[136] jsonlite_1.7.2 marray_1.66.0 R6_2.5.0
[139] sets_1.0-18 Hmisc_4.5-0 pillar_1.5.1
[142] htmltools_0.5.1.1 mime_0.10 glue_1.4.2
[145] fastmap_1.1.0 DT_0.17 BiocParallel_1.22.0
[148] codetools_0.2-18 fgsea_1.14.0 mvtnorm_1.1-1
[151] utf8_1.1.4 lattice_0.20-41 bslib_0.2.4
[154] curl_4.3 gtools_3.8.2 zip_2.1.1
[157] shinyjs_2.0.0 openxlsx_4.2.3 openssl_1.4.3
[160] survival_3.2-7 rmarkdown_2.7 munsell_0.5.0
[163] GetoptLong_1.0.5 GenomeInfoDbData_1.2.3 haven_2.3.1
[166] gtable_0.3.0