Correlate proteomic profiling with miRNA
Junyan Lu
2020-03-07
Last updated: 2020-03-13
Checks: 5 2
Knit directory: Proteomics/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.0). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20200227) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
- unnamed-chunk-3
To ensure reproducibility of the results, delete the cache directory correlateMIR_cache and re-run the analysis. To have workflowr automatically delete the cache directory prior to building the file, set delete_cache = TRUE when running wflow_build() or wflow_publish().
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .DS_Store
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/.DS_Store
Ignored: analysis/.Rhistory
Ignored: analysis/compareProteomicsRNAseq_cache/
Ignored: analysis/correlateCLLPD_cache/
Ignored: analysis/correlateGenomic_cache/
Ignored: analysis/correlateGenomic_noBlock_MCLL_cache/
Ignored: analysis/correlateGenomic_noBlock_UCLL_cache/
Ignored: analysis/correlateGenomic_noBlock_cache/
Ignored: analysis/correlateGenomic_removePC_cache/
Ignored: analysis/correlateMIR_cache/
Ignored: analysis/correlateMethylationCluster_cache/
Ignored: analysis/predictOutcome_cache/
Ignored: data/.DS_Store
Ignored: output/.DS_Store
Untracked files:
Untracked: analysis/correlateGenomic_noBlock.Rmd
Untracked: analysis/correlateGenomic_noBlock_MCLL.Rmd
Untracked: analysis/correlateGenomic_noBlock_UCLL.Rmd
Untracked: code/utils.R
Untracked: data/190909_CLL_prot_abund_med_norm.tsv
Untracked: data/190909_CLL_prot_abund_no_norm.tsv
Untracked: data/20190423_Proteom_submitted_samples_bereinigt.xlsx
Untracked: data/20191025_Proteom_submitted_samples_final.xlsx
Untracked: data/LUMOS/
Untracked: data/LUMOS_peptides/
Untracked: data/LUMOS_protAnnotation.csv
Untracked: data/SampleAnnotation_cleaned.xlsx
Untracked: data/facTab_IC50atLeast3New.RData
Untracked: data/gmts/
Untracked: data/mapEnsemble.txt
Untracked: data/mapSymbol.txt
Untracked: data/pyprophet_export_aligned.csv
Untracked: data/timsTOF_protAnnotation.csv
Untracked: output/LUMOS_processed.RData
Untracked: output/proteomic_LUMOS_20200227.RData
Untracked: output/proteomic_timsTOF_20200227.RData
Untracked: output/timsTOF_processed.RData
Unstaged changes:
Modified: analysis/correlateGenomic.Rmd
Modified: analysis/correlateMIR.Rmd
Modified: analysis/correlateMethylationCluster.Rmd
Modified: analysis/index.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the R Markdown and HTML files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view them.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| html | b8e0823 | Junyan Lu | 2020-03-10 | Build site. |
| Rmd | c8cb45c | Junyan Lu | 2020-03-10 | update analysis |
Test for important miRNA in CLL: miR15/16, miR34A
Are those three miRNA in our RNAseq dataset?
mirList <- c("MIR15","MIR16","MIR34A")
sapply(mirList, function(n) rowData(dds)$symbol[grepl(n,rowData(dds)$symbol)])Loading required package: DESeq2$MIR15
[1] "MIR153-1" "MIR15B" "MIR150" "MIR152" "MIR153-2" "MIR154"
[7] "MIR1537" "MIR1538" "MIR155HG" "MIR151A" "MIR1587" "MIR151B"
$MIR16
[1] "MIR16-2" "MIR16-1"
$MIR34A
[1] "MIR34AHG"miRNAs selected for test
mirSele <- c("MIR15B","MIR16-1","MIR16-2","MIR34AHG")
mirSele[1] "MIR15B" "MIR16-1" "MIR16-2" "MIR34AHG"Expression level of those 4 miRNAs
ddsMIR <- dds[rowData(dds)$symbol %in% mirSele,dds$PatID %in% colnames(protCLL)]
plotTab <- counts(ddsMIR,normalized = TRUE) %>% data.frame() %>% rownames_to_column("id") %>%
gather(key = "patID", value = "expr", -id) %>%
mutate(symbol = rowData(dds[id,])$symbol,
IGHV = protCLL[,patID]$IGHV.status,
trisomy12 = protCLL[,patID]$trisomy12)
ggplot(plotTab, aes(x=symbol, y = expr)) + geom_boxplot() + geom_point(aes(col = IGHV, shape = trisomy12)) +
scale_y_log10() + ylab("Normalized expression")Warning: Transformation introduced infinite values in continuous y-axis
Warning: Transformation introduced infinite values in continuous y-axisWarning: Removed 158 rows containing non-finite values (stat_boxplot).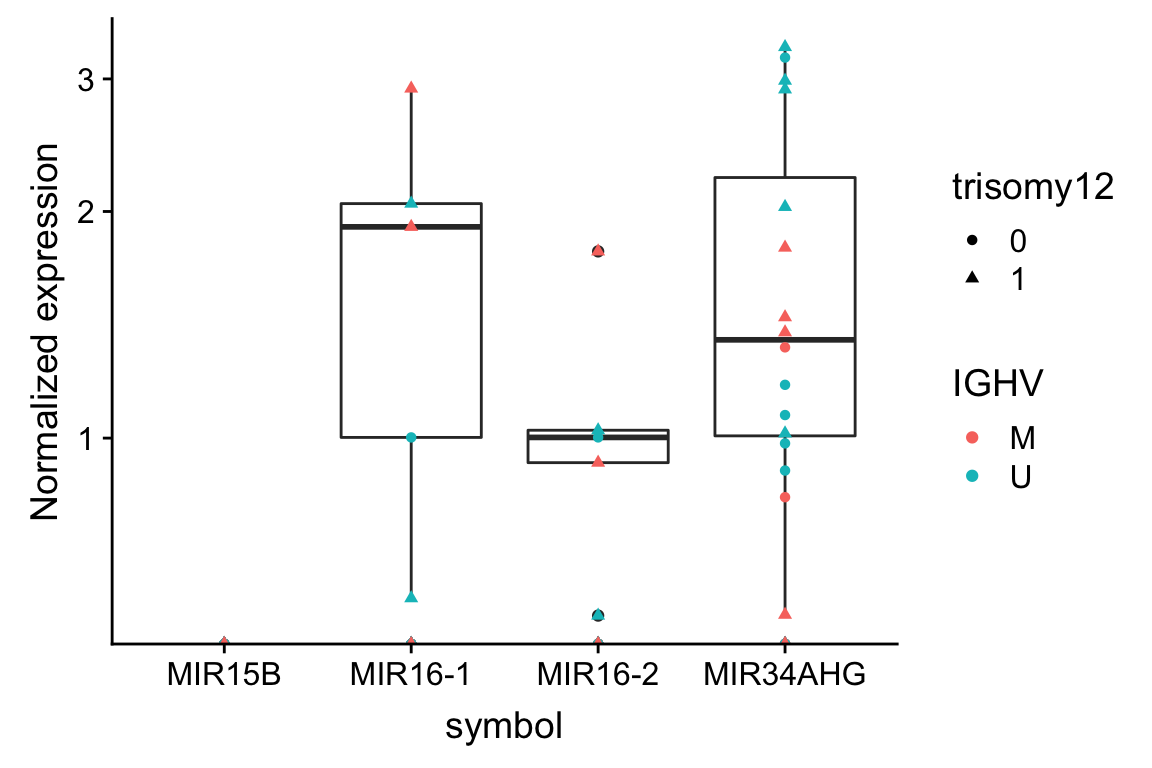
| Version | Author | Date |
|---|---|---|
| b8e0823 | Junyan Lu | 2020-03-10 |
The expressions are pretty low
Do the expressions of miRNA correlate with IGHV or trisomy12?
IGHV
testRes <- group_by(plotTab, symbol) %>% nest() %>%
mutate(m = map(data, ~wilcox.test(expr~IGHV, .))) %>%
mutate(res = map(m, broom::tidy)) %>%
unnest(res) %>%
select(symbol, p.value)
testRes# A tibble: 4 x 2
# Groups: symbol [4]
symbol p.value
<chr> <dbl>
1 MIR16-2 0.540
2 MIR15B NaN
3 MIR16-1 0.568
4 MIR34AHG 0.0636trisomy12
testRes <- group_by(plotTab, symbol) %>% nest() %>%
mutate(m = map(data, ~wilcox.test(expr~trisomy12, .))) %>%
mutate(res = map(m, broom::tidy)) %>%
unnest(res) %>%
select(symbol, p.value)
testRes# A tibble: 4 x 2
# Groups: symbol [4]
symbol p.value
<chr> <dbl>
1 MIR16-2 0.137
2 MIR15B NaN
3 MIR16-1 0.127
4 MIR34AHG 0.244No strong assocations
P-value histogram
hist(corTab$P.Value)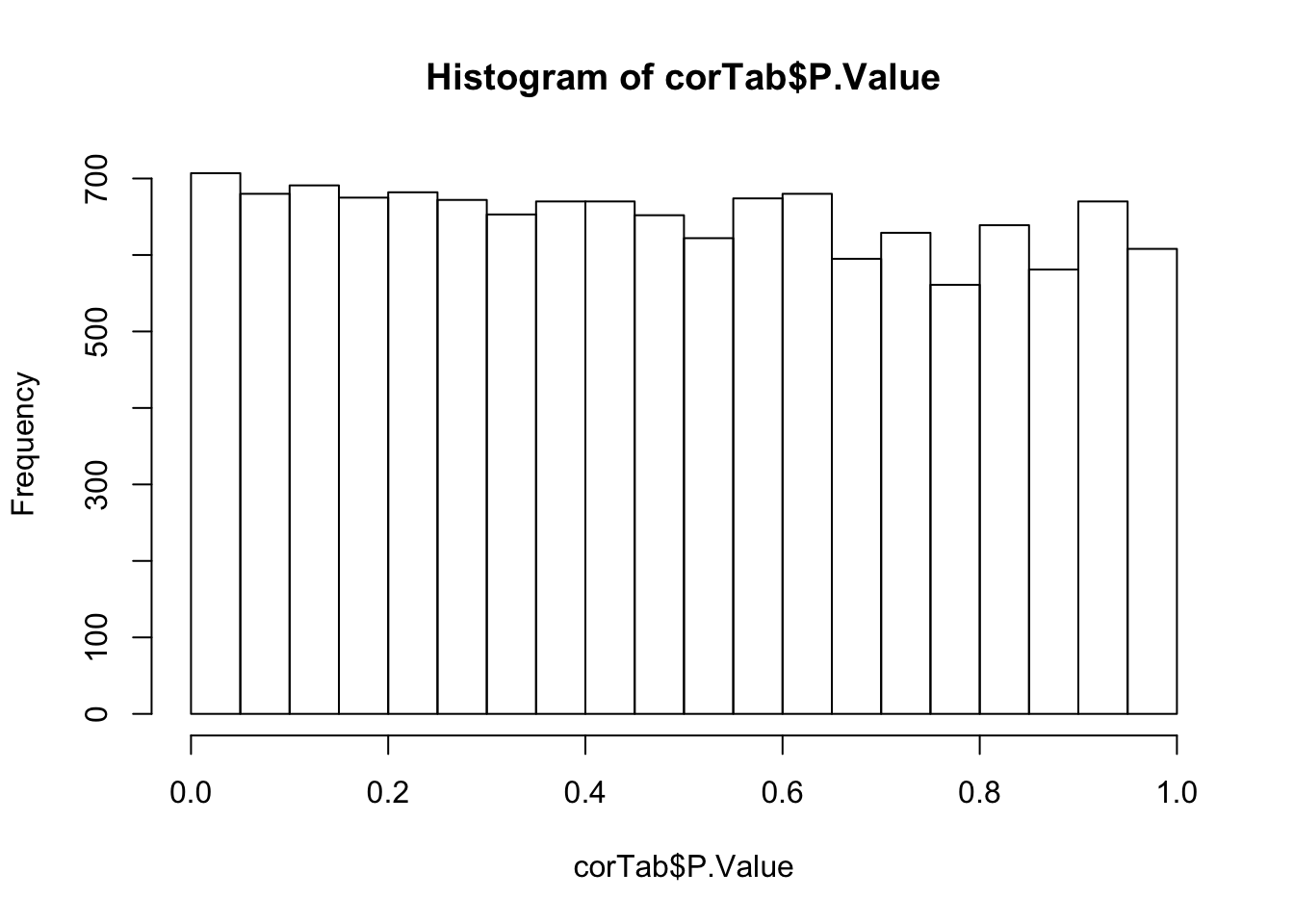 Overall, there are not many significant associations
Overall, there are not many significant associations
Table of significant associations (raw P value < 0.01, no assocation passed 10% FDR)
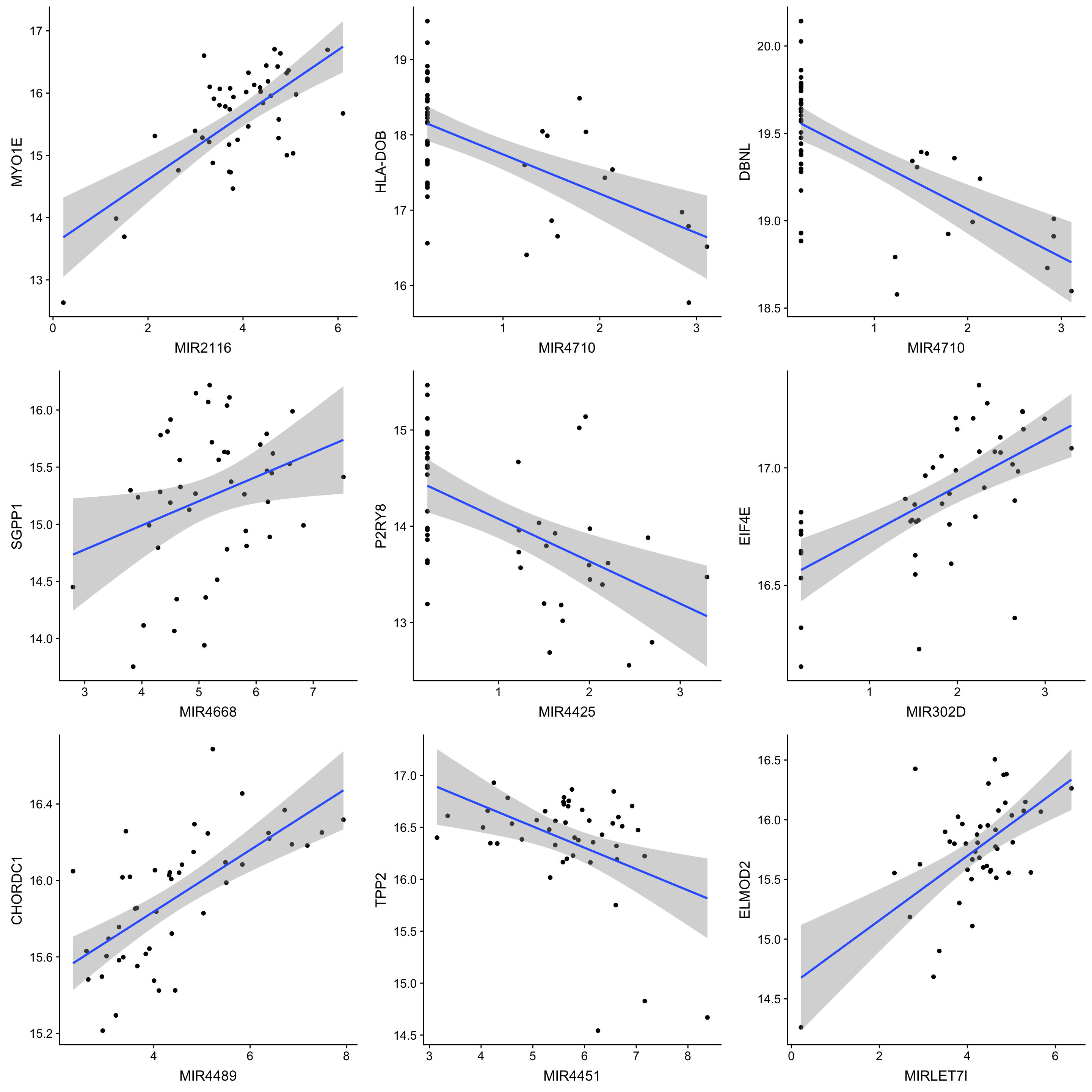
corTab.sig <- filter(corTab, P.Value < 0.01) %>% arrange(P.Value)
corTab.sig %>% select(geneSymbol, proteinSymbol, P.Value, adj.P.Val) %>%
arrange(P.Value) %>%
mutate_if(is.numeric, formatC, digits=2, format= "e") %>%
DT::datatable()Number of significant associations per miRNA (10% FDR)
sumTab <- corTab %>% filter(P.Value < 0.01) %>%
mutate(direction = ifelse(coef >0, "pos","neg")) %>%
group_by(geneSymbol, direction) %>% summarise(n=length(geneID)) %>%
arrange(desc(n))Bar plot
ggplot(sumTab, aes(x=geneSymbol, y = n, fill = direction)) + geom_bar(stat = "identity", position = position_dodge2()) +
theme(axis.text.x = element_text(angle =90, hjust=1, vjust=0.5))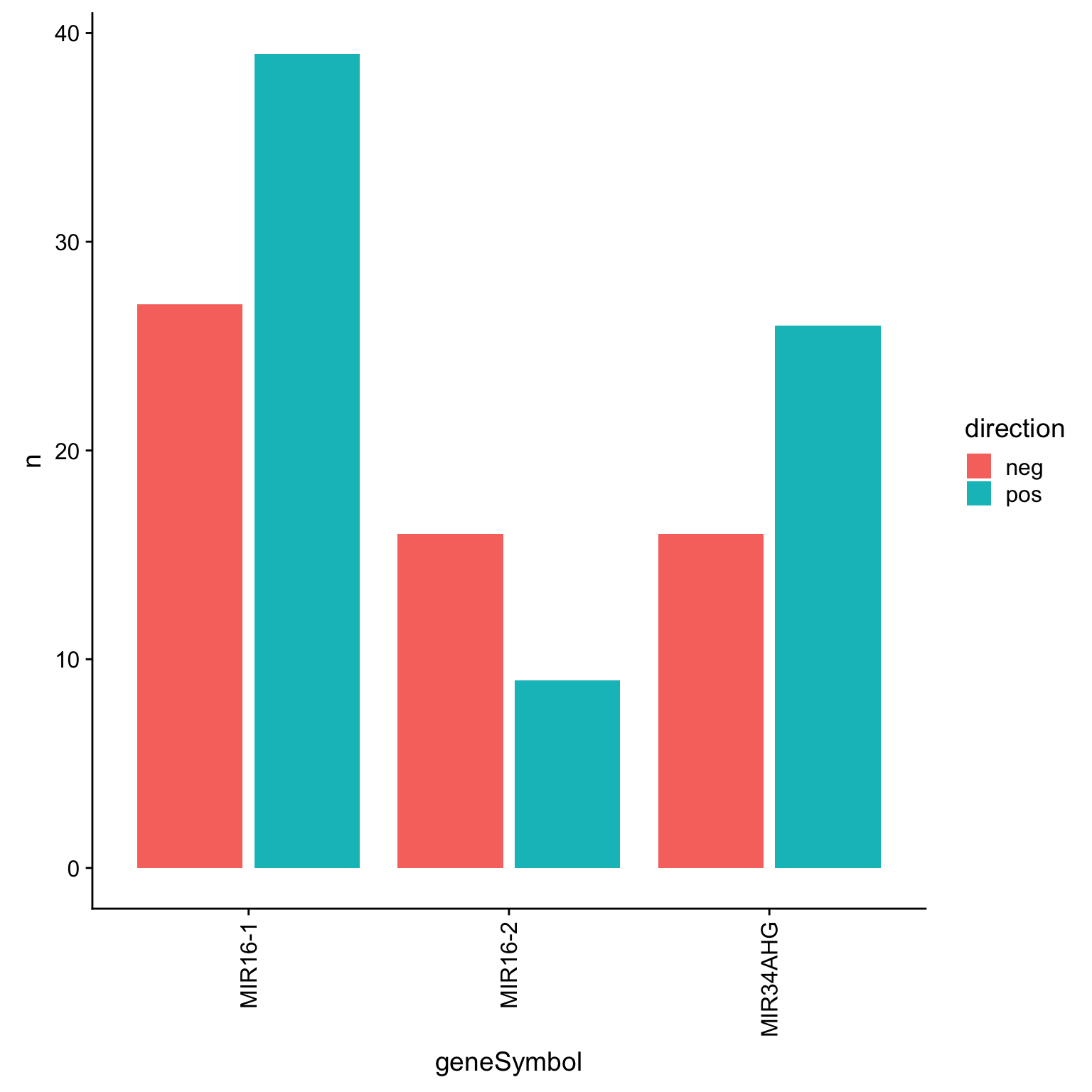
Correlation between all detected miRNA and protein expression
Get miRNA profile from RNAseq data
Not that the mRNAseq is optimized to capture protein coding genes, not miRNA.
ddsMIR <- dds[grepl("MIR",rowData(dds)$symbol),dds$PatID %in% colnames(protCLL)]
#remove gene that are not expressed
ddsMIR <- ddsMIR[rowSums(counts(ddsMIR, normalized = TRUE)) > 10,]
ddsMIR.vst <- varianceStabilizingTransformation(ddsMIR)
sds <- genefilter::rowSds(assay(ddsMIR.vst))
ddsMIR.vst <- ddsMIR.vst[sds > genefilter::shorth(sds),]
dim(ddsMIR.vst)[1] 228 46protSub <- protCLL[,colnames(ddsMIR.vst)]Here I use all the miRNAs that were detected by RNAseq and show variations of expression, if we have a more focused list, we may increase the power by reducing number of multilpe tests.
Calculate correlations between protein abundance and miRNA expression
In this test, I blocked for ighv and trisomy12, which means the test will identify correlations that are not confounded by IGHV and trisomy12
rnaMat <- assay(ddsMIR.vst)
proMat <- assays(protSub)[["count"]]
ighv <- protSub$IGHV.status
trisomy12 <- protSub$trisomy12
corTab <- lapply(rownames(rnaMat), function(i) {
rna <- rnaMat[i,]
designMat <- model.matrix(~1+ighv + trisomy12 + rna) #block for IGHV and trisomy12
fit <- lmFit(proMat, designMat)
fit2 <- eBayes(fit)
corRes <- topTable(fit2, number ="all", adjust.method = "BH", coef = "rna") %>% rownames_to_column("proteinID") %>%
mutate(geneID = i)
corRes
}) %>% bind_rows() %>% mutate(geneSymbol = rowData(dds[geneID,])$symbol,
proteinSymbol = rowData(protCLL[proteinID,])$hgnc_symbol) %>%
mutate(adj.P.All = p.adjust(P.Value, method = "BH"))P-value histogram
hist(corTab$P.Value)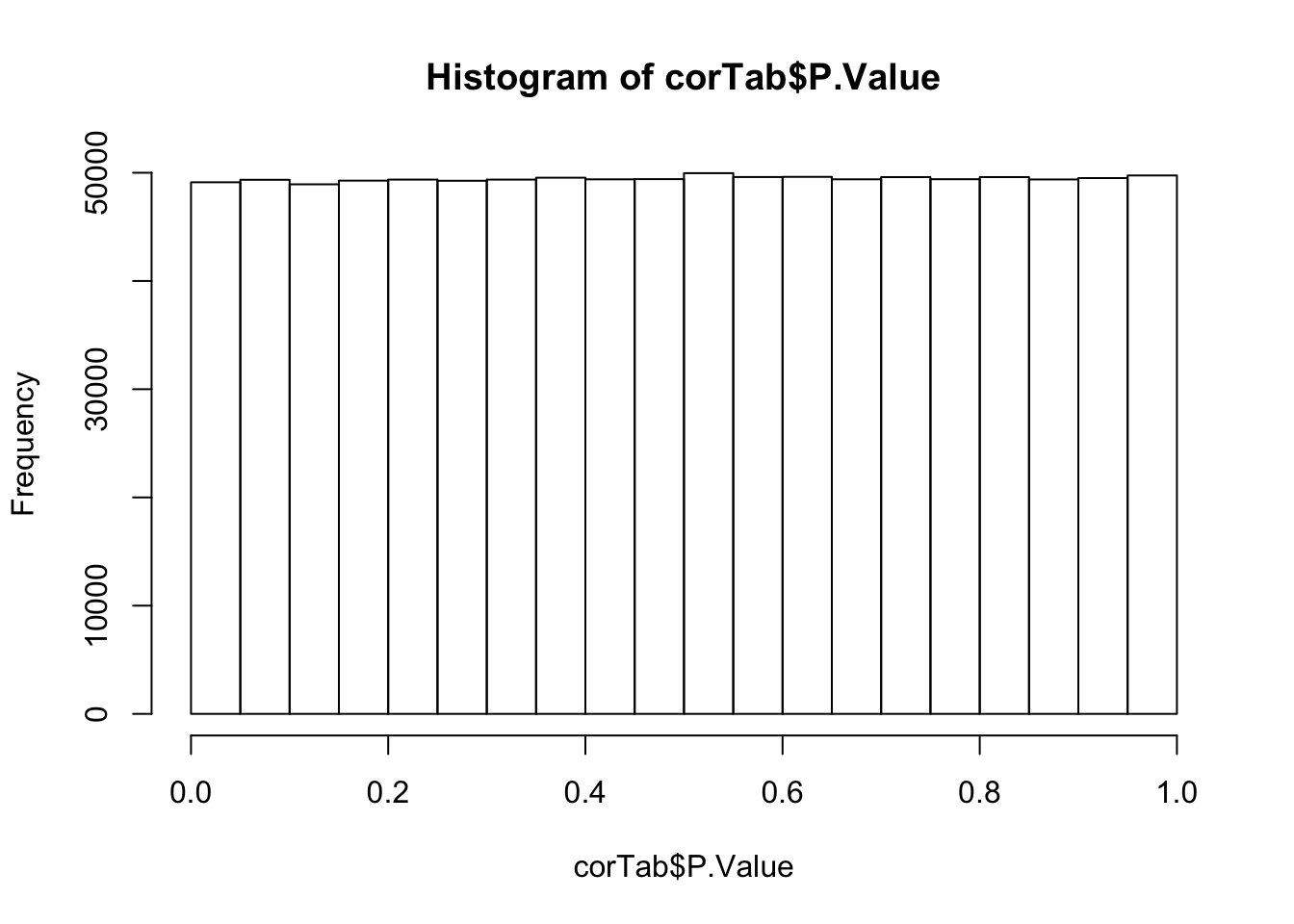 Overall, there are not many significant associations
Overall, there are not many significant associations
Table of significant associations (10% FDR)
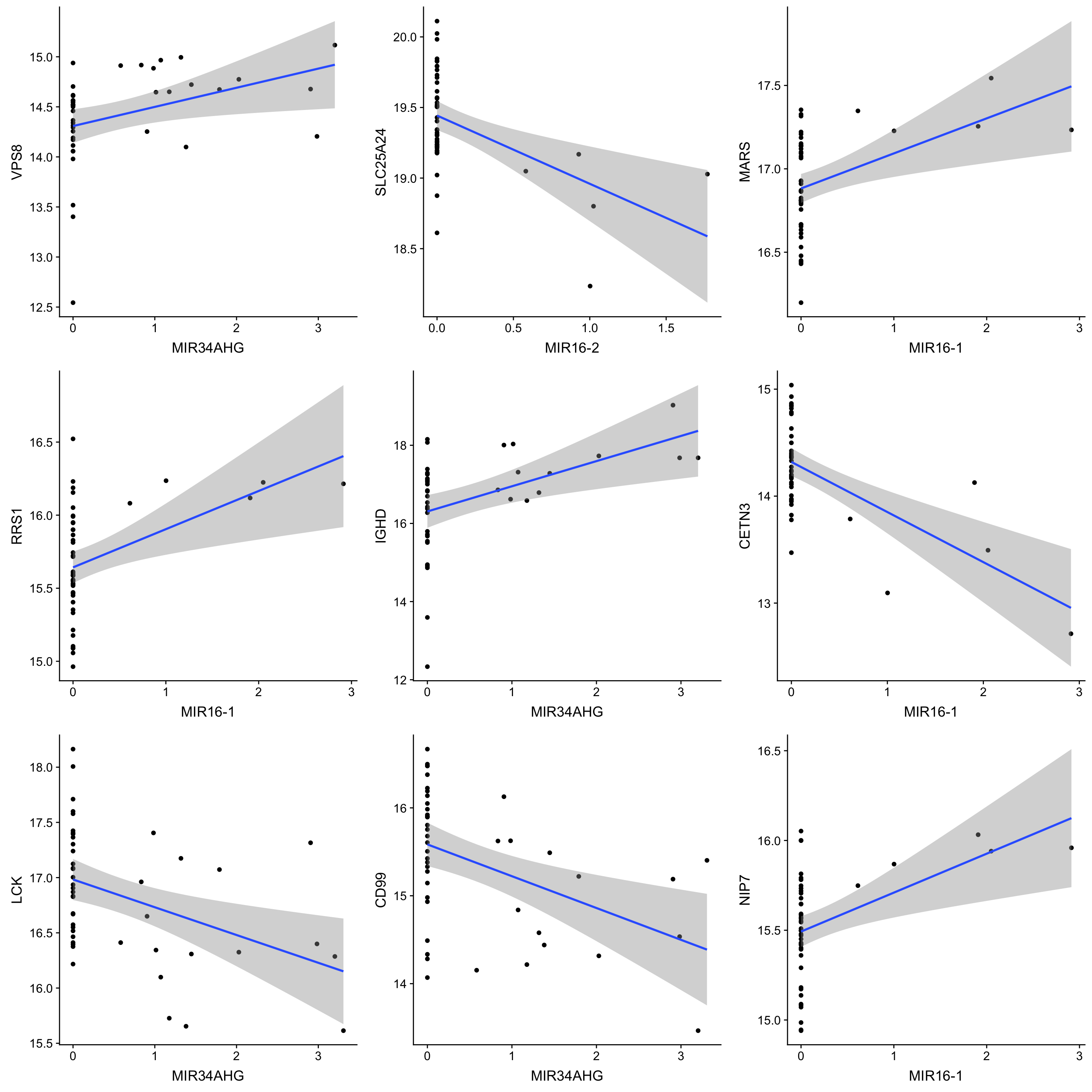
corTab.sig <- filter(corTab, adj.P.Val < 0.1) %>% arrange(P.Value)
corTab.sig %>% select(geneSymbol, proteinSymbol, P.Value, adj.P.Val) %>%
arrange(P.Value) %>%
mutate_if(is.numeric, formatC, digits=2, format= "e") %>%
DT::datatable()Number of significant associations per miRNA (10% FDR)
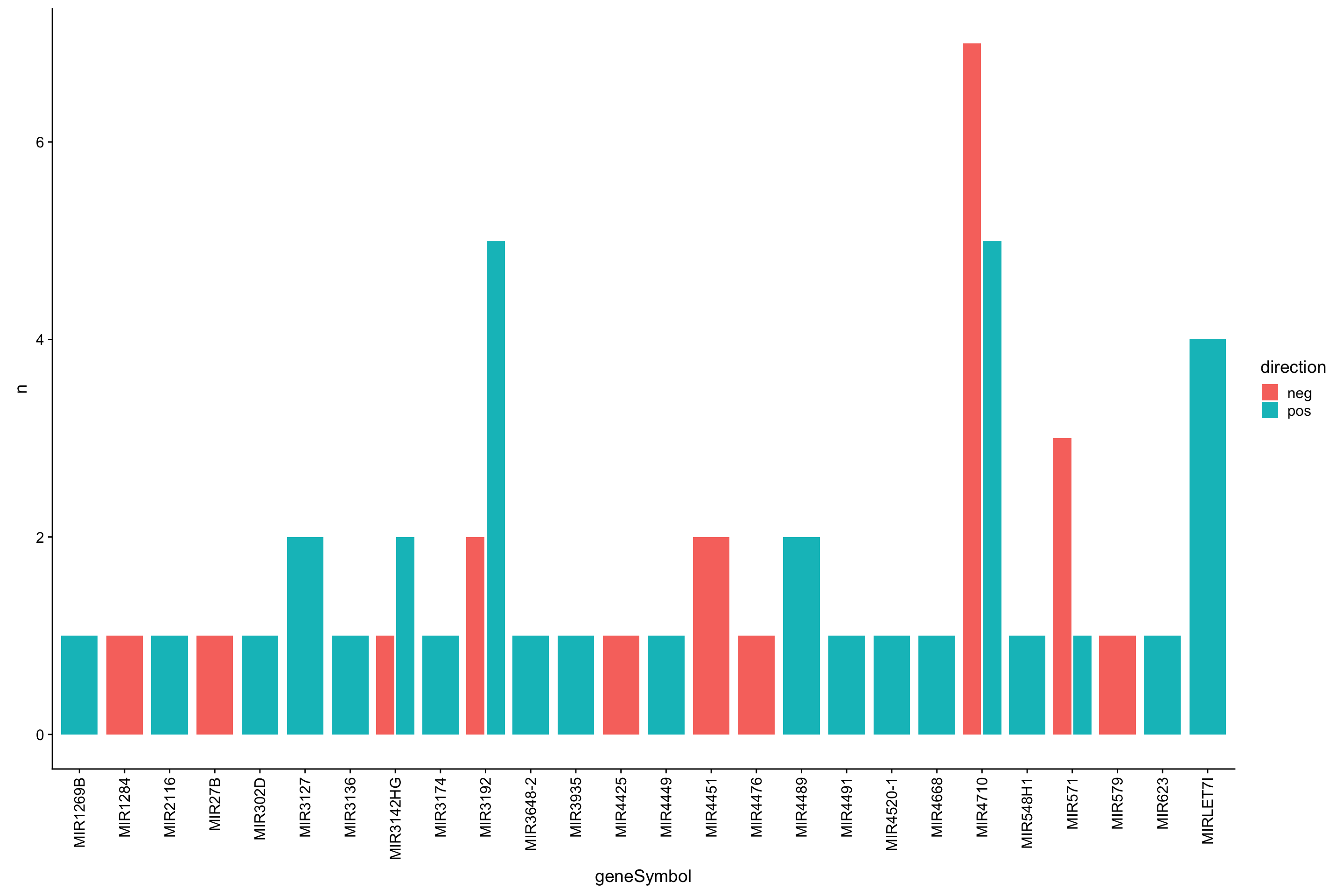
sumTab <- corTab %>% filter(adj.P.Val < 0.1) %>%
mutate(direction = ifelse(logFC >0, "pos","neg")) %>%
group_by(geneSymbol, direction) %>% summarise(n=length(geneID)) %>%
arrange(desc(n))Bar plot
ggplot(sumTab, aes(x=geneSymbol, y = n, fill = direction)) + geom_bar(stat = "identity", position = position_dodge2()) +
theme(axis.text.x = element_text(angle =90, hjust=1, vjust=0.5))